Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding
- PMID: 12451117
- PMCID: PMC6758766
- DOI: 10.1523/JNEUROSCI.22-23-10163.2002
Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding
Abstract
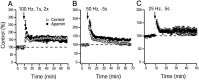
Activity-dependent changes in neuronal excitability and synaptic strength are thought to underlie memory encoding. In hippocampal CA1 neurons, small conductance Ca2+-activated K+ (SK) channels contribute to the afterhyperpolarization, affecting neuronal excitability. In the present study, we examined the effect of apamin-sensitive SK channels on the induction of hippocampal synaptic plasticity in response to a range of stimulation frequencies. In addition, the role of apamin-sensitive SK channels on hippocampal-dependent memory encoding and retention was also tested. The results show that blocking SK channels with apamin increased the excitability of hippocampal neurons and facilitated the induction of synaptic plasticity by shifting the modification threshold to lower frequencies. This facilitation was NMDA receptor (NMDAR) dependent and appeared to be postsynaptic. Mice treated with apamin demonstrated accelerated hippocampal-dependent spatial and nonspatial memory encoding. They required fewer trials to learn the location of a hidden platform in the Morris water maze and less time to encode object memory in an object-recognition task compared with saline-treated mice. Apamin did not influence long-term retention of spatial or nonspatial memory. These data support a role for SK channels in the modulation of hippocampal synaptic plasticity and hippocampal-dependent memory encoding.
Figures
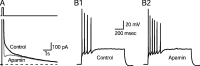




References
-
- Andreasen M, Lambert JD. The excitability of CA1 pyramidal cell dendrites is modulated by a local Ca2+-dependent K+-conductance. Brain Res. 1995;698:193–203. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. - PubMed
-
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett. 1998;253:91–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
