Changes in conformation and subcellular distribution of alpha4beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras
- PMID: 12451118
- PMCID: PMC6758762
- DOI: 10.1523/JNEUROSCI.22-23-10172.2002
Changes in conformation and subcellular distribution of alpha4beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras
Abstract
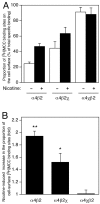
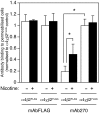
Chronic exposure to nicotine, as occurs during tobacco smoking, is one of several factors that have been reported to cause an upregulation of neuronal nicotinic acetylcholine receptors (nAChRs). Here, the influence of both chronic exposure to nicotine (10 microm, 24 hr) and the coexpression of subunit chimeras has been examined in cultured cell lines expressing recombinant alpha4beta2 nAChRs, a major nicotinic receptor subtype expressed in the mammalian brain. Evidence is presented which demonstrates that both chronic exposure to nicotine and the coexpression of subunit chimeras upregulates levels of receptor expressed on the cell surface. Immunoblotting data indicate that neither chronic nicotine treatment nor coexpressed subunit partners greatly affect the level of total subunit protein. This finding, together with radioligand and antibody binding studies conducted on both intact and permeabilized cells, reveals that receptor upregulation corresponds to an increase in the proportion of total receptor expressed on the cell surface. It is also apparent that nicotine-induced nAChR upregulation is very strongly dependent on subunit composition and subunit domains. An important aspect of this study is that direct evidence has been obtained indicating that both chronic exposure to nicotine and coexpressed subunit partners can influence subunit conformation. The influence of chronic nicotine treatment on subunit folding may help to explain the phenomenon of nicotine-induced receptor upregulation. The finding that subunit conformation can be influenced by coassembled subunit partners is in agreement with models of receptor assembly which propose that subunit folding continues after initial subunit-subunit interactions.
Figures





Similar articles
-
Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression.Brain Res. 2006 Oct 3;1113(1):94-109. doi: 10.1016/j.brainres.2006.06.084. Epub 2006 Aug 30. Brain Res. 2006. PMID: 16942759
-
Rare human nicotinic acetylcholine receptor α4 subunit (CHRNA4) variants affect expression and function of high-affinity nicotinic acetylcholine receptors.J Pharmacol Exp Ther. 2014 Mar;348(3):410-20. doi: 10.1124/jpet.113.209767. Epub 2014 Jan 2. J Pharmacol Exp Ther. 2014. PMID: 24385388 Free PMC article.
-
Molecular characterization of Dalpha6 and Dalpha7 nicotinic acetylcholine receptor subunits from Drosophila: formation of a high-affinity alpha-bungarotoxin binding site revealed by expression of subunit chimeras.J Neurochem. 2004 Jul;90(2):479-89. doi: 10.1111/j.1471-4159.2004.02499.x. J Neurochem. 2004. PMID: 15228604
-
Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure.Curr Drug Targets CNS Neurol Disord. 2002 Aug;1(4):359-85. doi: 10.2174/1568007023339184. Curr Drug Targets CNS Neurol Disord. 2002. PMID: 12769610 Review.
-
Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery.AAPS J. 2009 Mar;11(1):167-77. doi: 10.1208/s12248-009-9090-7. Epub 2009 Mar 12. AAPS J. 2009. PMID: 19280351 Free PMC article. Review.
Cited by
-
Examination of the Involvement of Cholinergic-Associated Genes in Nicotine Behaviors in European and African Americans.Nicotine Tob Res. 2017 Apr 1;19(4):417-425. doi: 10.1093/ntr/ntw200. Nicotine Tob Res. 2017. PMID: 27613895 Free PMC article.
-
bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse.Br J Pharmacol. 2011 May;163(2):346-57. doi: 10.1111/j.1476-5381.2011.01220.x. Br J Pharmacol. 2011. PMID: 21232049 Free PMC article.
-
Neurotransmitter receptors as central regulators of pancreatic cancer.Future Oncol. 2010 Feb;6(2):221-8. doi: 10.2217/fon.09.171. Future Oncol. 2010. PMID: 20146581 Free PMC article. Review.
-
Genome-wide expression analysis reveals diverse effects of acute nicotine exposure on neuronal function-related genes and pathways.Front Psychiatry. 2011 Mar 8;2:5. doi: 10.3389/fpsyt.2011.00005. eCollection 2011. Front Psychiatry. 2011. PMID: 21556275 Free PMC article.
-
Cell autonomy, receptor autonomy, and thermodynamics in nicotine receptor up-regulation.Biochem Pharmacol. 2007 Oct 15;74(8):1145-54. doi: 10.1016/j.bcp.2007.06.040. Epub 2007 Jun 30. Biochem Pharmacol. 2007. PMID: 17662697 Free PMC article.
References
-
- Bencherif M, Fowler K, Lukas R, Lippiello PM. Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther. 1995;275:987–994. - PubMed
-
- Benwell MEM, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. - PubMed
-
- Chen D, Dang H, Patrick JW. Contributions of N-linked glycosylation to the expression of a functional α7-nicotinic receptor in Xenopus oocytes. J Neurochem. 1998;70:349–357. - PubMed
-
- Cooper ST, Millar NS. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor α7 subunit. J Neurochem. 1997;68:2140–2151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
