Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons
- PMID: 12451121
- PMCID: PMC6758763
- DOI: 10.1523/JNEUROSCI.22-23-10201.2002
Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons
Abstract
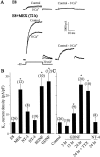
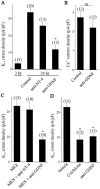
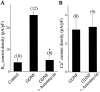
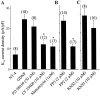
The functional expression of large-conductance Ca2+-activated K+ (K(Ca)) channels in lumbar motoneurons (LMNs) of the developing chick embryo is regulated in part by interactions with striated muscle target tissues. Here we show that the functional expression of K(Ca) channels in LMNs developing in vitro can be stimulated by application of a skeletal muscle extract (MEX) or by coculture with hindlimb myotubes. A similar stimulation of K(Ca) channels in vitro can be produced by the trophic factors glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor but not by neurotrophin (NT)-3 or NT-4. The actions of MEX and hindlimb myotubes are blocked by a GDNF-neutralizing antiserum. Moreover, injection of this same antiserum into the embryonic hindlimb reduced the functional expression of K(Ca) channels in vivo to levels seen in LMNs deprived of interactions with the hindlimb. The effects of GDNF on K(Ca) channel expression in LMNs require 24 hr of continuous exposure to reach maximum and are blocked by the translation inhibitor anisomycin, indicating the need for synthesis of new proteins. GDNF actions are also blocked by the farnesyl transferase inhibitor manumycin, suggesting a role for Ras in the actions of GDNF. Finally, the actions of GDNF are inhibited by PP2, an inhibitor of Src family tyrosine kinases, and by LY29003, an inhibitor of phosphatidylinositol 3 kinases, but not by PD98059, an inhibitor of the Erk signaling cascade. None of these treatments alter expression of voltage-activated Ca2+ channels. Thus, the actions of GDNF on LMN K(Ca) channel expression appear to use a transduction pathway similar to that used for regulation of apoptosis.
Figures
Similar articles
-
Activity- and target-dependent regulation of large-conductance Ca2+-activated K+ channels in developing chick lumbar motoneurons.J Neurosci. 2002 Jan 1;22(1):73-81. doi: 10.1523/JNEUROSCI.22-01-00073.2002. J Neurosci. 2002. PMID: 11756490 Free PMC article.
-
Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord.J Neurosci. 1998 Oct 1;18(19):7903-11. doi: 10.1523/JNEUROSCI.18-19-07903.1998. J Neurosci. 1998. PMID: 9742158 Free PMC article.
-
Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons.J Neurosci. 1999 Nov 1;19(21):9160-9. doi: 10.1523/JNEUROSCI.19-21-09160.1999. J Neurosci. 1999. PMID: 10531419 Free PMC article.
-
Expression of K(Ca) channels in identified populations of developing vertebrate neurons: role of neurotrophic factors and activity.J Physiol Paris. 2003 Jan;97(1):49-58. doi: 10.1016/j.jphysparis.2003.10.006. J Physiol Paris. 2003. PMID: 14706690 Review.
-
Role of cell-cell interactions in the developmental regulation of Ca2+-activated K+ currents in vertebrate neurons.J Neurobiol. 1998 Oct;37(1):23-36. doi: 10.1002/(sici)1097-4695(199810)37:1<23::aid-neu3>3.0.co;2-a. J Neurobiol. 1998. PMID: 9777730 Review.
Cited by
-
Electrophysiological properties of BK channels in Xenopus motor nerve terminals.J Physiol. 2004 May 15;557(Pt 1):207-28. doi: 10.1113/jphysiol.2003.060509. Epub 2004 Mar 26. J Physiol. 2004. PMID: 15047773 Free PMC article.
-
[Research advances of artemin].Zhongguo Fei Ai Za Zhi. 2011 Oct;14(10):790-800. doi: 10.3779/j.issn.1009-3419.2011.10.05. Zhongguo Fei Ai Za Zhi. 2011. PMID: 22008109 Free PMC article. Review. Chinese. No abstract available.
-
Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling.J Neurosci. 2009 Mar 25;29(12):3852-64. doi: 10.1523/JNEUROSCI.0213-09.2009. J Neurosci. 2009. PMID: 19321782 Free PMC article.
-
Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain.J Neurochem. 2009 Jul;110(1):352-62. doi: 10.1111/j.1471-4159.2009.06138.x. Epub 2009 Apr 30. J Neurochem. 2009. PMID: 19457113 Free PMC article.
-
Temporally tuned neuronal differentiation supports the functional remodeling of a neuronal network in Drosophila.Proc Natl Acad Sci U S A. 2012 Mar 27;109(13):E748-56. doi: 10.1073/pnas.1114710109. Epub 2012 Mar 5. Proc Natl Acad Sci U S A. 2012. PMID: 22393011 Free PMC article.
References
-
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Alonso G, Assenmacher Retrograde axoplasmic transport of neurosecretory material. An immunocytochemical and electron-microscopic study of transected axons in normal and colchicine-treated rats. Cell Tissue Res. 1983;233:183–196. - PubMed
-
- Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors–implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. - PubMed
-
- Becker E, Soler RM, Yuste VJ, Gine E, Sanz-Rodriguez C, Egea J, Martin-Zanca D, Comella JX. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J Neurosci. 1998;18:7903–7911. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
