Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions
- PMID: 12451126
- PMCID: PMC6758747
- DOI: 10.1523/JNEUROSCI.22-23-10251.2002
Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions
Abstract
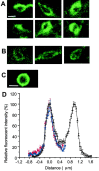
GABA transporter subtype 1 (GAT1) molecules were counted near GABAergic synapses, to a resolution of approximately 0.5 microm. Fusions between GAT1 and green fluorescent protein (GFP) were tested in heterologous expression systems, and a construct was selected that shows function, expression level, and trafficking similar to that of wild-type (WT) GAT1. A strain of knock-in mice was constructed that expresses this mGAT1-GFP fusion in place of the WT GAT1 gene. The pattern of fluorescence in brain slices agreed with previous immunocytochemical observations. [3H]GABA uptake, synaptic electrophysiology, and subcellular localization of the mGAT1-GFP construct were also compared with WT mice. Quantitative fluorescence microscopy was used to measure the density of mGAT1-GFP at presynaptic structures in CNS preparations from the knock-in mice. Fluorescence measurements were calibrated with transparent beads and gels that have known GFP densities. Surface biotinylation defined the fraction of transporters on the surface versus those in the nearby cytoplasm. The data show that the presynaptic boutons of GABAergic interneurons in cerebellum and hippocampus have a membrane density of 800-1300 GAT1 molecules per square micrometer, and the axons that connect boutons have a linear density of 640 GAT1 molecules per micrometer. A cerebellar basket cell bouton, a pinceau surrounding a Purkinje cell axon, and a cortical chandelier cell cartridge carry 9000, 7.8 million, and 430,000 GAT1 molecules, respectively; 61-63% of these molecules are on the surface membrane. In cultures from hippocampus, the set of fluorescent cells equals the set of GABAergic interneurons. Knock-in mice carrying GFP fusions of membrane proteins provide quantitative data required for understanding the details of synaptic transmission in living neurons.
Figures





References
-
- Acsady L, Gorcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73:317–334. - PubMed
-
- Ahn J, Mundigl O, Muth TR, Rudnick G, Caplan MJ. Polarized expression of GABA transporters in Madin-Darby canine kidney cells and cultured hippocampal neurons. J Biol Chem. 1996;271:6917–6924. - PubMed
-
- Altman J, Bayer S . . Development of the cerebellar system in relation to its evolution, structure, and functions. CRC; Boca Raton, FL: 1997.
-
- Anglister L, Stiles JR, Salpeter MM. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994;12:783–794. - PubMed
-
- Barakat L, Bordey A. GAT-1 and reversible GABA transporter in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–1419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-030549/NS/NINDS NIH HHS/United States
- R29 DA010509/DA/NIDA NIH HHS/United States
- R01 NS011756/NS/NINDS NIH HHS/United States
- R01 MH061468/MH/NIMH NIH HHS/United States
- R37 NS030549/NS/NINDS NIH HHS/United States
- DA-010509/DA/NIDA NIH HHS/United States
- R01 DA009121/DA/NIDA NIH HHS/United States
- R01 DA010509/DA/NIDA NIH HHS/United States
- NS-11756/NS/NINDS NIH HHS/United States
- MH-49176/MH/NIMH NIH HHS/United States
- MH-61468/MH/NIMH NIH HHS/United States
- DA-09121/DA/NIDA NIH HHS/United States
- R01 NS030549/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
