Quantal size and variation determined by vesicle size in normal and mutant Drosophila glutamatergic synapses
- PMID: 12451127
- PMCID: PMC6758758
- DOI: 10.1523/JNEUROSCI.22-23-10267.2002
Quantal size and variation determined by vesicle size in normal and mutant Drosophila glutamatergic synapses
Abstract
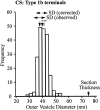
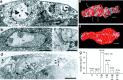
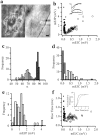
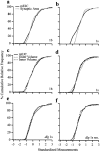
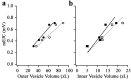
Quantal size and variation at chemical synapses could be determined presynaptically by the amount of neurotransmitter released from synaptic vesicles or postsynaptically by the number of receptors available for activation. We investigated these possibilities at Drosophila glutamatergic neuromuscular synapses formed by two separate motor neurons innervating the same muscle cell. At wild-type synapses of the two neurons we found a difference in quantal size corresponding to a difference in mean synaptic vesicle volume. The same finding applied to two mutants (dlg and lap) in which synaptic vesicle size was altered. Quantal variances at wild-type and mutant synapses were similar and could be accounted for by variation in vesicular volume. The linear relationship between quantal size and vesicular volume for several different genotypes indicates that glutamate is regulated homeostatically to the same intravesicular concentration in all cases. Thus functional differences in synaptic strength among glutamatergic neurons of Drosophila result in part from intrinsic differences in vesicle size.
Figures

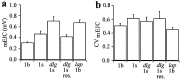
References
-
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. - PubMed
-
- Atwood HL, Tse FW. Physiological aspects of presynaptic inhibition. Adv Neural Sci. 1993;1:19–65.
-
- Atwood HL, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
-
- Atwood HL, Karunanithi S, Georgiou J, Charlton MP. Strength of synaptic transmission at neuromuscular junctions of crustaceans and insects in relation to calcium entry. Invert Neurosci. 1997;3:81–87. - PubMed
-
- Atwood HL, Karunanithi S, Wong K, Marin L, Stewart BA. Determinants of quantal size at Drosophila synapses: bouton type, vesicle size, and the size of the postsynaptic receptor patch. Soc Neurosci Abstr. 1999;25:187.11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
