Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease
- PMID: 12451130
- PMCID: PMC6758736
- DOI: 10.1523/JNEUROSCI.22-23-10302.2002
Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease
Abstract
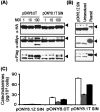
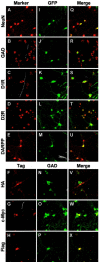
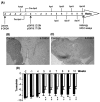
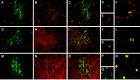
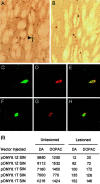
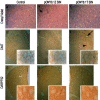
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the selective loss of dopaminergic neurons in the substantia nigra. This loss leads to complete dopamine depletion in the striatum and severe motor impairment. It has been demonstrated previously that a lentiviral vector system based on equine infectious anemia virus (EIAV) gives rise to highly efficient and sustained transduction of neurons in the rat brain. Therefore, a dopamine replacement strategy using EIAV has been investigated as a treatment in the 6-hydroxydopamine (6-OHDA) animal model of PD. A self-inactivating EIAV minimal lentiviral vector that expresses tyrosine hydroxylase (TH), aromatic amino acid dopa decarboxylase (AADC), and GTP cyclohydrolase 1 (CH1) in a single transcription unit has been generated. In cultured striatal neurons transduced with this vector, TH, AADC, and CH1 proteins can all be detected. After stereotactic delivery into the dopamine-denervated striatum of the 6-OHDA-lesioned rat, sustained expression of each enzyme and effective production of catecholamines were detected, resulting in significant reduction of apomorphine-induced motor asymmetry compared with control animals (p < 0.003). Expression of each enzyme in the striatum was observed for up to 5 months after injection. These data indicate that the delivery of three catecholaminergic synthetic enzymes by a single lentiviral vector can achieve functional improvement and thus open the potential for the use of this vector for gene therapy of late-stage PD patients.
Figures


Similar articles
-
Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson's disease.Hum Gene Ther. 2000 Jul 20;11(11):1509-19. doi: 10.1089/10430340050083243. Hum Gene Ther. 2000. PMID: 10945765
-
Coexpression of tyrosine hydroxylase, GTP cyclohydrolase I, aromatic amino acid decarboxylase, and vesicular monoamine transporter 2 from a helper virus-free herpes simplex virus type 1 vector supports high-level, long-term biochemical and behavioral correction of a rat model of Parkinson's disease.Hum Gene Ther. 2004 Dec;15(12):1177-96. doi: 10.1089/hum.2004.15.1177. Hum Gene Ther. 2004. PMID: 15684695 Free PMC article.
-
Role of aromatic L-amino acid decarboxylase for dopamine replacement by genetically modified fibroblasts in a rat model of Parkinson's disease.J Neurochem. 1997 Nov;69(5):2055-63. doi: 10.1046/j.1471-4159.1997.69052055.x. J Neurochem. 1997. PMID: 9349551
-
Recent progress in gene therapy for Parkinson's disease.Curr Mol Med. 2012 Dec;12(10):1311-8. doi: 10.2174/156652412803833580. Curr Mol Med. 2012. PMID: 22834832 Review.
-
Catecholamines and Parkinson's disease: tyrosine hydroxylase (TH) over tetrahydrobiopterin (BH4) and GTP cyclohydrolase I (GCH1) to cytokines, neuromelanin, and gene therapy: a historical overview.J Neural Transm (Vienna). 2024 Jun;131(6):617-630. doi: 10.1007/s00702-023-02673-y. Epub 2023 Aug 28. J Neural Transm (Vienna). 2024. PMID: 37638996 Review.
Cited by
-
Synthetic biology with surgical precision: targeted reengineering of signaling proteins.Cell Signal. 2012 Oct;24(10):1899-908. doi: 10.1016/j.cellsig.2012.05.012. Epub 2012 Jun 1. Cell Signal. 2012. PMID: 22664341 Free PMC article. Review.
-
Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study.Hum Gene Ther. 2017 Jan;28(1):99-111. doi: 10.1089/hum.2016.117. Epub 2016 Sep 26. Hum Gene Ther. 2017. PMID: 27710144 Free PMC article. Clinical Trial.
-
An update on gene therapy in Parkinson's disease.Curr Neurol Neurosci Rep. 2011 Aug;11(4):362-70. doi: 10.1007/s11910-011-0197-8. Curr Neurol Neurosci Rep. 2011. PMID: 21479996 Review.
-
Gene Therapy Approach with an Emphasis on Growth Factors: Theoretical and Clinical Outcomes in Neurodegenerative Diseases.Mol Neurobiol. 2022 Jan;59(1):191-233. doi: 10.1007/s12035-021-02555-y. Epub 2021 Oct 15. Mol Neurobiol. 2022. PMID: 34655056 Free PMC article. Review.
-
Gene-Based Therapeutics for Parkinson's Disease.Biomedicines. 2022 Jul 26;10(8):1790. doi: 10.3390/biomedicines10081790. Biomedicines. 2022. PMID: 35892690 Free PMC article. Review.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Ariano MA, Sortwell CE, Ray M, Altemus KL, Sibley DR, Levine MS. Agonist-induced morphologic decrease in cellular D1A dopamine receptor staining. Synapse. 1997;27:313–321. - PubMed
-
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. - PubMed
-
- Barker RA, Dunnett SB. Functional integration of neural grafts in Parkinson's disease. Nat Neurosci. 1999;2:1047–1048. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
