The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons
- PMID: 12451134
- PMCID: PMC6758749
- DOI: 10.1523/JNEUROSCI.22-23-10346.2002
The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons
Abstract
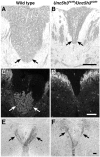
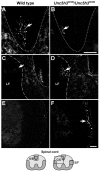
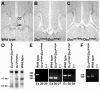
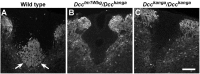
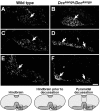
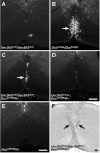
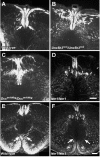
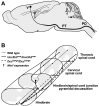
Migrating axons require the correct presentation of guidance molecules, often at multiple choice points, to find their target. Netrin 1, a bifunctional cue involved in both attracting and repelling axons, is involved in many cell migration and axon pathfinding processes in the CNS. The netrin 1 receptor DCC and its Caenorhabditis elegans homolog UNC-40 have been implicated in directing the guidance of axons toward netrin sources, whereas the C. elegans UNC-6 receptor, UNC-5 is necessary for migrations away from UNC-6. However, a role of vertebrate UNC-5 homologs in axonal migration has not been demonstrated. We demonstrate that the Unc5h3 gene product, shown previously to regulate cerebellar granule cell migrations, also controls the guidance of the corticospinal tract, the major tract responsible for coordination of limb movements. Furthermore, we show that corticospinal tract fibers respond differently to loss of UNC5H3. In addition, we observe corticospinal tract defects in mice homozygous for a spontaneous mutation that truncates the Dcc transcript. Postnatal day 0 netrin 1 mutant mice also demonstrate corticospinal tract abnormalities. Last, interactions between the Dcc and Unc5h3 mutations were observed in gene dosage experiments. This is the first evidence of an involvement in axon guidance for any member of the vertebrate unc-5 family and confirms that both the cellular and axonal guidance functions of C. elegans unc-5 have been conserved in vertebrates.
Figures




References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
-
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. - PubMed
-
- Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics. 1994;19:525–531. - PubMed
-
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. - PubMed
-
- Colavita A, Culotti JG. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol. 1998;194:72–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
