Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord
- PMID: 12451138
- PMCID: PMC6758765
- DOI: 10.1523/JNEUROSCI.22-23-10388.2002
Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord
Abstract
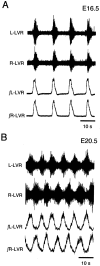
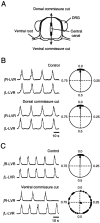
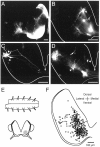
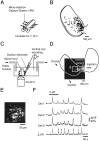
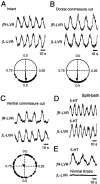
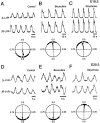
The basic neuronal networks generating coordinated rhythmic motor activity, such as left-right alternate limb movement during locomotion in mammals, are located in the spinal cord. In rat fetuses, the spatial pattern of the rhythmic activity between the left and right sides is synchronous at and shortly after rhythmogenesis before the pattern becomes alternate by birth. The neuronal mechanisms underlying these developmental changes in the left-right coordination were examined in isolated spinal cord preparations. Calcium imaging of commissural neurons at the early fetal stages revealed that the intracellular Ca2+ concentration of the commissural neurons was elevated by bath-application of 5-hydroxytryptamine (5-HT) in synchrony with the simultaneously recorded rhythmic activity of the ventral root, suggesting that the commissural neurons mediate the left-right coordination of the rhythmic activity from onset of the rhythmogenesis. Using a longitudinal split-bath setup, we show that the synchronicity in pattern of the rhythmic activity is the result of excitatory connections being formed via commissural neurons between the rhythm-generating networks located in the left and right spinal cord. During this period, such connections were found to be mediated by excitatory synaptic transmission via GABA(A) receptors. When the pattern of rhythmic activity became left-right alternate at later fetal stages, these connections, still via GABA(A) receptors, were mediating reciprocal inhibition between the two sides. Nearer birth, glycine receptors took over this role. Our results reveal the nature of the neuronal mechanisms forming the basis of the left-right coordination of rhythmic motor activity during prenatal development.
Figures



Similar articles
-
Rhythmic motor activity in thin transverse slice preparations of the fetal rat spinal cord.J Neurophysiol. 2004 Jul;92(1):648-52. doi: 10.1152/jn.01029.2003. Epub 2004 Mar 17. J Neurophysiol. 2004. PMID: 15028747
-
Developmental changes in 5-hydroxytryptamine-induced rhythmic activity in the spinal cord of rat fetuses in vitro.Neurosci Lett. 2001 Jul 6;307(1):1-4. doi: 10.1016/s0304-3940(01)01913-9. Neurosci Lett. 2001. PMID: 11516560
-
Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network.J Neurosci. 1997 Dec 15;17(24):9433-47. doi: 10.1523/JNEUROSCI.17-24-09433.1997. J Neurosci. 1997. PMID: 9390999 Free PMC article.
-
The role of inhibitory neurotransmission in locomotor circuits of the developing mammalian spinal cord.Acta Physiol (Oxf). 2009 Oct;197(2):83-97. doi: 10.1111/j.1748-1716.2009.02020.x. Epub 2009 Jul 16. Acta Physiol (Oxf). 2009. PMID: 19673737 Review.
-
Developmental changes in rhythmic spinal neuronal activity in the rat fetus.Prog Brain Res. 2004;143:49-55. doi: 10.1016/s0079-6123(03)43005-7. Prog Brain Res. 2004. PMID: 14653150 Review.
Cited by
-
Single-Cell Reconstruction of Emerging Population Activity in an Entire Developing Circuit.Cell. 2019 Oct 3;179(2):355-372.e23. doi: 10.1016/j.cell.2019.08.039. Epub 2019 Sep 26. Cell. 2019. PMID: 31564455 Free PMC article.
-
Maturation of the GABAergic transmission in normal and pathologic motoneurons.Neural Plast. 2011;2011:905624. doi: 10.1155/2011/905624. Epub 2011 Jul 20. Neural Plast. 2011. PMID: 21785735 Free PMC article. Review.
-
Roles of Retinoic Acid Signaling in Shaping the Neuronal Architecture of the Developing Amphioxus Nervous System.Mol Neurobiol. 2018 Jun;55(6):5210-5229. doi: 10.1007/s12035-017-0727-8. Epub 2017 Sep 5. Mol Neurobiol. 2018. PMID: 28875454
-
Primitive roles for inhibitory interneurons in developing frog spinal cord.J Neurosci. 2004 Jun 23;24(25):5840-8. doi: 10.1523/JNEUROSCI.1633-04.2004. J Neurosci. 2004. PMID: 15215306 Free PMC article.
-
Hop Mice Display Synchronous Hindlimb Locomotion and a Ventrally Fused Lumbar Spinal Cord Caused by a Point Mutation in Ttc26.eNeuro. 2022 Mar 14;9(2):ENEURO.0518-21.2022. doi: 10.1523/ENEURO.0518-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35210288 Free PMC article.
References
-
- Batschelet E. Circular statistics in biology (Sibson R, Cohen JE, eds). Academic; New York: 1981.
-
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J Neurophysiol. 1982;47:961–975. - PubMed
-
- Buchanan JT, Cohen AH. Activities of identified interneurons, motoneurons, and muscle fibers during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J Neurophysiol. 1982;47:948–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
