Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts
- PMID: 12451140
- PMCID: PMC6758746
- DOI: 10.1523/JNEUROSCI.22-23-10408.2002
Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts
Abstract
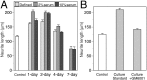
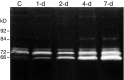
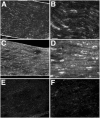
Injury to peripheral nerve initiates a degenerative process that converts the denervated nerve from a suppressive environment to one that promotes axonal regeneration. We investigated the role of matrix metalloproteinases (MMPs) in this degenerative process and whether effective predegenerated nerve grafts could be produced in vitro. Rat peripheral nerve explants were cultured for 1-7 d in various media, and their neurite-promoting activity was assessed by cryoculture assay, in which neurons are grown directly on nerve sections. The neurite-promoting activity of cultured nerves increased rapidly and, compared with uncultured nerve, a maximum increase of 72% resulted by 2 d of culture in the presence of serum. Remarkably, the neurite-promoting activity of short-term cultured nerves was also significantly better than nerves degenerated in vivo. We examined whether in vitro degeneration is MMP dependent and found that the MMP inhibitor N-[(2R)-2(hydroxamidocarbonylmethyl)-4-methylpantanoyl]-l-tryptophan methylamide primarily blocked the degenerative increase in neurite-promoting activity. In the absence of hematogenic macrophages, MMP-9 was trivial, whereas elevated MMP-2 expression and activation paralleled the increase in neurite-promoting activity. MMP-2 immunoreactivity localized to Schwann cells and the endoneurium and colocalized with gelatinolytic activity as demonstrated by in situ zymography. Finally, in vitro predegenerated nerves were tested as acellular grafts and, compared with normal acellular nerve grafts, axonal ingress in vivo was approximately doubled. We conclude that Schwann cell expression of MMP-2 plays a principal role in the degenerative process that enhances the regeneration-promoting properties of denervated nerve. Combined with their low immunogenicity, acellular nerve grafts activated by in vitro predegeneration may be a significant advancement for clinical nerve allografting.
Figures



Similar articles
-
MMP-2 and MMP-9 increase the neurite-promoting potential of schwann cell basal laminae and are upregulated in degenerated nerve.Mol Cell Neurosci. 2000 Aug;16(2):157-67. doi: 10.1006/mcne.2000.0859. Mol Cell Neurosci. 2000. PMID: 10924258
-
Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan.J Neurosci. 2001 Aug 15;21(16):6206-13. doi: 10.1523/JNEUROSCI.21-16-06206.2001. J Neurosci. 2001. PMID: 11487643 Free PMC article.
-
[Effect of pre-degeneration of peripheral nerves on plasticity of cultivated Schwann cells and their cell number in vitro].Handchir Mikrochir Plast Chir. 1999 Nov;31(6):367-72. doi: 10.1055/s-1999-13554. Handchir Mikrochir Plast Chir. 1999. PMID: 10637725 German.
-
Nerve physiology: mechanisms of injury and recovery.Hand Clin. 2013 Aug;29(3):317-30. doi: 10.1016/j.hcl.2013.04.002. Hand Clin. 2013. PMID: 23895713 Free PMC article. Review.
-
Molecular mechanisms of cellular interactions in peripheral nerve regeneration.Curr Opin Neurol. 2001 Oct;14(5):635-9. doi: 10.1097/00019052-200110000-00013. Curr Opin Neurol. 2001. PMID: 11562576 Review.
Cited by
-
TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve.Mol Cell Neurosci. 2006 Mar;31(3):407-15. doi: 10.1016/j.mcn.2005.10.011. Epub 2005 Nov 16. Mol Cell Neurosci. 2006. PMID: 16297636 Free PMC article.
-
Acute- and late-phase matrix metalloproteinase (MMP)-9 activity is comparable in female and male rats after peripheral nerve injury.J Neuroinflammation. 2018 Mar 20;15(1):89. doi: 10.1186/s12974-018-1123-7. J Neuroinflammation. 2018. PMID: 29558999 Free PMC article.
-
Matrix metalloproteinase 7 promoted Schwann cell migration and myelination after rat sciatic nerve injury.Mol Brain. 2019 Dec 2;12(1):101. doi: 10.1186/s13041-019-0516-6. Mol Brain. 2019. PMID: 31791378 Free PMC article.
-
Uridine 5'-triphosphate promotes in vitro Schwannoma cell migration through matrix metalloproteinase-2 activation.PLoS One. 2014 Jun 6;9(6):e98998. doi: 10.1371/journal.pone.0098998. eCollection 2014. PLoS One. 2014. PMID: 24905332 Free PMC article.
-
A consistent, quantifiable, and graded rat lumbosacral spinal cord injury model.J Neurotrauma. 2015 Jun 15;32(12):875-92. doi: 10.1089/neu.2013.3321. Epub 2015 Mar 12. J Neurotrauma. 2015. PMID: 25313633 Free PMC article.
References
-
- Bain JR. Peripheral nerve allografting: review of the literature with relevance to composite tissue transplantation. Transplant Proc. 1998;30:2762–2767. - PubMed
-
- Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. - PubMed
-
- Brück W, Brück Y, Maruschak B, Friede RL. Mechanisms of macrophage recruitment in Wallerian degeneration. Acta Neuropathol (Berl) 1995;89:363–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
