Resetting intrinsic purinergic modulation of neural activity: an associative mechanism?
- PMID: 12451145
- PMCID: PMC6758752
- DOI: 10.1523/JNEUROSCI.22-23-10461.2002
Resetting intrinsic purinergic modulation of neural activity: an associative mechanism?
Abstract
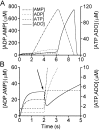
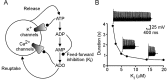
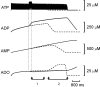
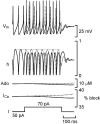
The purines, ATP and adenosine, control the rundown and termination of swimming in the Xenopus embryo. This intrinsic purinergic modulation, unavoidably present during every swimming episode, could lead to stereotyped inflexible behavior and consequently could jeopardize the survival of the embryo. To explore whether this control system can exhibit adaptability, I have used a minimal simulation in which a model neuron released ATP that (1) inhibited K+ currents and (2) was converted by ectonucleotidases to adenosine, which then inhibited Ca2+ currents. The model neuron exhibited an accommodating spike train controlled by the actions of ATP and adenosine. Feedforward inhibition by the upstream metabolite ADP of the ecto-5'-nucleotidase that converts AMP to adenosine introduced adaptability and allowed the resetting of spike accommodation. The strength of feedforward inhibition determined the extent to which resetting could occur. I have tested these predictions by examining swimming in the real embryo. The rundown of swimming was reset in a manner similar to that predicted by the single-neuron model. By blocking the purinoceptors, I have demonstrated that resetting in the embryo is attributable to the actions of the purines and results from feedforward inhibition of adenosine production. The resetting of rundown in the motor systems can be reformulated as an associative mechanism in which the temporal coincidence of two stimuli can prolong network activity if they fall within a particular time window. The length of the time window and the magnitude of the prolongation of neural activity both depend on the strength of the feedforward ADP-mediated inhibition of the ecto-5'-nucleotidase.
Figures





Similar articles
-
Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos.Nature. 1996 Sep 19;383(6597):259-63. doi: 10.1038/383259a0. Nature. 1996. PMID: 8805702
-
Modulation of K(+) currents in Xenopus spinal neurons by p2y receptors: a role for ATP and ADP in motor pattern generation.J Physiol. 2002 May 1;540(Pt 3):843-50. doi: 10.1113/jphysiol.2001.013192. J Physiol. 2002. PMID: 11986373 Free PMC article.
-
A role for potassium currents in the generation of the swimming motor pattern of Xenopus embryos.J Neurophysiol. 1994 Jul;72(1):337-48. doi: 10.1152/jn.1994.72.1.337. J Neurophysiol. 1994. PMID: 7965018
-
Ectonucleoside triphosphate diphosphohydrolases and ecto-5'-nucleotidase in purinergic signaling: how the field developed and where we are now.Purinergic Signal. 2021 Mar;17(1):117-125. doi: 10.1007/s11302-020-09755-6. Epub 2020 Dec 17. Purinergic Signal. 2021. PMID: 33336318 Free PMC article. Review.
-
History of ectonucleotidases and their role in purinergic signaling.Biochem Pharmacol. 2021 May;187:114322. doi: 10.1016/j.bcp.2020.114322. Epub 2020 Nov 6. Biochem Pharmacol. 2021. PMID: 33161020 Review.
Cited by
-
Positive feedback loops sustain repeating bursts in neuronal circuits.J Biol Phys. 2011 Jun;37(3):317-45. doi: 10.1007/s10867-010-9210-8. Epub 2010 Dec 16. J Biol Phys. 2011. PMID: 22654180 Free PMC article.
-
Plasticity of purine release during cerebral ischemia: clinical implications?J Cell Mol Med. 2003 Oct-Dec;7(4):362-75. doi: 10.1111/j.1582-4934.2003.tb00239.x. J Cell Mol Med. 2003. PMID: 14754505 Free PMC article. Review.
-
From decision to action: Detailed modelling of frog tadpoles reveals neuronal mechanisms of decision-making and reproduces unpredictable swimming movements in response to sensory signals.PLoS Comput Biol. 2021 Dec 13;17(12):e1009654. doi: 10.1371/journal.pcbi.1009654. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34898604 Free PMC article.
-
Neural control and modulation of swimming speed in the larval zebrafish.Neuron. 2014 Aug 6;83(3):692-707. doi: 10.1016/j.neuron.2014.06.032. Epub 2014 Jul 24. Neuron. 2014. PMID: 25066084 Free PMC article.
-
Beyond the wiring diagram: signalling through complex neuromodulator networks.Philos Trans R Soc Lond B Biol Sci. 2010 Aug 12;365(1551):2363-74. doi: 10.1098/rstb.2010.0105. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20603357 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
