Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice
- PMID: 12451149
- PMCID: PMC6758751
- DOI: 10.1523/JNEUROSCI.22-23-10494.2002
Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice
Abstract
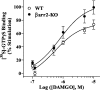
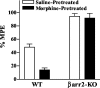
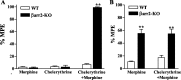
Morphine induces antinociception by activating mu opioid receptors (muORs) in spinal and supraspinal regions of the CNS. (Beta)arrestin-2 (beta)arr2), a G-protein-coupled receptor-regulating protein, regulates the muOR in vivo. We have shown previously that mice lacking (beta)arr2 experience enhanced morphine-induced analgesia and do not become tolerant to morphine as determined in the hot-plate test, a paradigm that primarily assesses supraspinal pain responsiveness. To determine the general applicability of the (beta)arr2-muOR interaction in other neuronal systems, we have, in the present study, tested (beta)arr2 knock-out ((beta)arr2-KO) mice using the warm water tail-immersion paradigm, which primarily assesses spinal reflexes to painful thermal stimuli. In this test, the (beta)arr2-KO mice have greater basal nociceptive thresholds and markedly enhanced sensitivity to morphine. Interestingly, however, after a delayed onset, they do ultimately develop morphine tolerance, although to a lesser degree than the wild-type (WT) controls. In the (beta)arr2-KO but not WT mice, morphine tolerance can be completely reversed with a low dose of the classical protein kinase C (PKC) inhibitor chelerythrine. These findings provide in vivo evidence that the muOR is differentially regulated in diverse regions of the CNS. Furthermore, although (beta)arr2 appears to be the most prominent and proximal determinant of muOR desensitization and morphine tolerance, in the absence of this mechanism, the contributions of a PKC-dependent regulatory system become readily apparent.
Figures




References
-
- Allen RM, Dykstra LA. The competitive NMDA receptor antagonist LY235959 modulates the progression of morphine tolerance in rats. Psychopharmacology (Berl) 1999;142:209–214. - PubMed
-
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. - PubMed
-
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000b;408:720–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
