Gangliosides inhibit the development from monocytes to dendritic cells
- PMID: 12452834
- PMCID: PMC1906548
- DOI: 10.1046/j.1365-2249.2002.02006.x
Gangliosides inhibit the development from monocytes to dendritic cells
Abstract
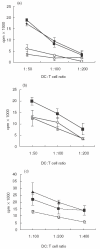
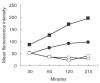
Dendritic cell (DC) development and function is critical in the initiation phase of any antigen-specific immune response against tumours. Impaired function of DC is one explanation as to how tumours escape immunosurveillance. In the presence of various soluble tumour-related factors DC precursors lose their ability to differentiate into mature DC and to activate T cells. Gangliosides are glycosphingolipids shed by tumours of neuroectodermal origin such as melanoma and neuroblastoma. In this investigation we address the question of whether gangliosides suppress the development and function of monocyte-derived DC in vitro. In the presence of gangliosides, the monocytic DC precursors showed increased adherence, cell spreading and a reduced number of dendrites. The expression of MHC class II molecules, co-stimulatory molecules and the GM-CSF receptor (CD116) on the ganglioside-treated DC was significantly reduced. Furthermore, the function of ganglioside-treated DC was impaired as observed in endocytosis, chemotactic and T cell proliferation assays. In contrast to monocytic DC precursors, mature DC were unaffected even when higher doses of gangliosides were added to the culture. With regard to their carbohydrate structure, five different gangliosides (GM2, GM3, GD2, GD3, GT1b), which are typically shed by melanoma and neuroblastoma, were tested for their ability to suppress DC development and function. Suppression was induced by GM2, but not by the other gangliosides. These data suggest that certain gangliosides impair DC precursors, implying a possible mechanism for tumour escape.
Figures



Similar articles
-
Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function.Cancer Res. 2001 Jan 1;61(1):363-9. Cancer Res. 2001. PMID: 11196188
-
Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis.J Immunol. 2003 Apr 1;170(7):3488-94. doi: 10.4049/jimmunol.170.7.3488. J Immunol. 2003. PMID: 12646609
-
Different mechanisms are involved in apoptosis induced by melanoma gangliosides on human monocyte-derived dendritic cells.Glycobiology. 2009 Jun;19(6):576-82. doi: 10.1093/glycob/cwp015. Epub 2009 Feb 24. Glycobiology. 2009. PMID: 19240275 Free PMC article.
-
Neuroblastoma and dendritic cell function.Semin Pediatr Surg. 2004 Feb;13(1):61-71. doi: 10.1053/j.sempedsurg.2003.09.009. Semin Pediatr Surg. 2004. PMID: 14765372 Review.
-
Role of tumor-associated gangliosides in cancer progression.Biochimie. 2003 Mar-Apr;85(3-4):455-63. doi: 10.1016/s0300-9084(03)00006-3. Biochimie. 2003. PMID: 12770784 Review.
Cited by
-
The Sweet Side of Immune Evasion: Role of Glycans in the Mechanisms of Cancer Progression.Front Oncol. 2016 Mar 9;6:54. doi: 10.3389/fonc.2016.00054. eCollection 2016. Front Oncol. 2016. PMID: 27014629 Free PMC article. Review.
-
The role of human milk fats in shaping neonatal development and the early life gut microbiota.Microbiome Res Rep. 2023 Mar 31;2(2):8. doi: 10.20517/mrr.2023.09. eCollection 2023. Microbiome Res Rep. 2023. PMID: 38047278 Free PMC article. Review.
-
The immunomodulating roles of glycoproteins in epithelial ovarian cancer.Front Biosci (Elite Ed). 2012 Jan 1;4(2):631-50. doi: 10.2741/405. Front Biosci (Elite Ed). 2012. PMID: 22201900 Free PMC article. Review.
-
Tissue micro array analysis of ganglioside N-glycolyl GM3 expression and signal transducer and activator of transcription (STAT)-3 activation in relation to dendritic cell infiltration and microvessel density in non-small cell lung cancer.BMC Cancer. 2009 Jun 11;9:180. doi: 10.1186/1471-2407-9-180. BMC Cancer. 2009. PMID: 19519895 Free PMC article.
-
Signaling defects in anti-tumor T cells.Immunol Rev. 2008 Apr;222:192-205. doi: 10.1111/j.1600-065X.2008.00606.x. Immunol Rev. 2008. PMID: 18364003 Free PMC article. Review.
References
-
- Esche C, Lokshin A, Shurin GV, et al. Tumors other immune targets: dendritic cells. J Leuk Biol. 1999;66:336–44. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1999;392:245–52. - PubMed
-
- Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune response. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1995;170:101–10. - PubMed
-
- Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996;170:111–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

