The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester
- PMID: 12452835
- PMCID: PMC1906537
- DOI: 10.1046/j.1365-2249.2002.01992.x
The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester
Abstract
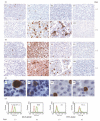
Gp-340 is a glycoprotein belonging to the scavenger receptor cysteine rich (SRCR) group B family. It binds to host immune components such as lung surfactant protein D (SP-D). Recent studies found that gp-340 interacts directly with pathogenic microorganisms and induces their aggregation, suggesting its involvement in innate immunity. In order to investigate further its potential immune functions in the appropriate cell lines, the expression of gp-340 in four conventional immune cell lines (U937, HL60, Jurkat, Raji), and two innate immune-related epithelial cell lines (A549 derived from lung and AGS from stomach), was examined by RT-PCR and immunohistochemistry. The resting immune cell lines showed weak or no gp-340 mRNA expression; while the two epithelial cell lines expressed gp-340 at much higher level, which was differentially regulated by phorbol myristate acetate (PMA) treatment. In the A549 cells, gp-340 was up-regulated along with the PMA-induced proinflammatory expression of both IL-6 and IL-8. In AGS cells, PMA down-regulation of gp-340 was seen in parallel with an up-regulation of the two mature gastric epithelial specific proteins TFF1 (trefoil factor 1) and TFF2, which are implicated as markers of terminal differentiation. Analysis of the distribution of gp-340, together with the TFFs and SP-D in normal lung and gastric mucosa, supported further our in vitro data. We conclude that the differential regulation of gp-340 in the two epithelial cell lines by PMA indicates that gp-340 s involvement in mucosal defence and growth of epithelial cells may vary at different body locations and during different stages of epithelial differentiation.
Figures





Similar articles
-
Expression of human intestinal trefoil factor in malignant cells and its regulation by oestrogen in breast cancer cells.J Pathol. 1997 Aug;182(4):404-13. doi: 10.1002/(SICI)1096-9896(199708)182:4<404::AID-PATH875>3.0.CO;2-0. J Pathol. 1997. PMID: 9306961
-
Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues.J Pathol. 2002 Aug;197(5):582-8. doi: 10.1002/path.1147. J Pathol. 2002. PMID: 12210076
-
Expression of TFF1, TFF2 and TFF3 in gastric cancer.Int J Oncol. 2002 Sep;21(3):655-9. Int J Oncol. 2002. PMID: 12168114
-
Trefoil factors and human gastric cancer (review).Int J Mol Med. 2003 Jul;12(1):3-9. Int J Mol Med. 2003. PMID: 12792801 Review.
-
Trefoil peptides: a newly recognized family of epithelial mucin-associated molecules.Am J Physiol. 1993 Aug;265(2 Pt 1):G205-13. doi: 10.1152/ajpgi.1993.265.2.G205. Am J Physiol. 1993. PMID: 8368306 Review.
Cited by
-
Increased expression of deleted in malignant brain tumors (DMBT1) gene in precancerous gastric lesions: Findings from human and animal studies.Oncotarget. 2017 Jul 18;8(29):47076-47089. doi: 10.18632/oncotarget.16792. Oncotarget. 2017. PMID: 28423364 Free PMC article.
-
Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin.Int J Mol Sci. 2024 Jan 26;25(3):1515. doi: 10.3390/ijms25031515. Int J Mol Sci. 2024. PMID: 38338794 Free PMC article.
-
Respiratory Deleted in Malignant Brain Tumours 1 (DMBT1) levels increase during lung maturation and infection.Clin Exp Immunol. 2008 Jan;151(1):123-9. doi: 10.1111/j.1365-2249.2007.03528.x. Epub 2007 Nov 7. Clin Exp Immunol. 2008. PMID: 17991292 Free PMC article.
-
Suppression of IL-8 production in gastric epithelial cells by MUC1 mucin and peroxisome proliferator-associated receptor-γ.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 15;303(6):G765-74. doi: 10.1152/ajpgi.00023.2012. Epub 2012 Jul 5. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22766852 Free PMC article.
-
DMBT1 expression is down-regulated in breast cancer.BMC Cancer. 2004 Aug 9;4:46. doi: 10.1186/1471-2407-4-46. BMC Cancer. 2004. PMID: 15301691 Free PMC article.
References
-
- Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev. 2000;173:66–78. - PubMed
-
- Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–54. - PubMed
-
- Holmskov U, Lawson P, Teisner B, et al. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J Biol Chem. 1997;272:13743–9. - PubMed
-
- Bodian DL, Skonier JE, Bowen MA, et al. Identification of residues in CD6 which are critical for ligand binding. Biochemistry. 1997;36:2637–41. - PubMed
-
- Bauskin AR, Franken DR, Eberspaecher U, et al. Characterization of human zona pellucida glycoproteins. Mol Hum Reprod. 1999;5:534–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

