Murine glomerular mesangial cell uptake of apoptotic cells is inefficient and involves serum-mediated but complement-independent mechanisms
- PMID: 12452836
- PMCID: PMC1906558
- DOI: 10.1046/j.1365-2249.2002.01998.x
Murine glomerular mesangial cell uptake of apoptotic cells is inefficient and involves serum-mediated but complement-independent mechanisms
Abstract
An increased number of apoptotic bodies have been detected in glomeruli of non-nephritic kidneys of C1q-deficient mice. In these mice an in vivo impaired uptake of apoptotic cells by peritoneal macrophages was also demonstrated. Here we investigated whether C1q plays a role in the in vitro clearance of apoptotic cells by glomerular mesangial cells. Phagocytosis was assessed using a novel flow cytometric assay that was validated by immunofluorescence studies. The uptake of apoptotic cells by mesangial cells, measured as percentage of mesangial cells ingesting apoptotic cells, was approximately 25%, 10% and 10% for a T cell lymphoma line (RMA), thymocytes and neutrophils, respectively. The uptake reached a plateau phase after 3 h, was specific for apoptotic cells and was mediated by serum but not by complement components C1q or C3. The phagocytosis of apoptotic cells was significantly inhibited by Arg-Gly-Asp-Ser (RGDS), a peptide capable of blocking the interaction of thrombospondin with CD36 or the vitronectin receptor. Pretreatment of the mesangial cells with dexamethasone (200 nm) but not with LPS increased the uptake markedly. These findings indicate that murine mesangial cells are capable of taking up syngeneic apoptotic cells, although much less efficiently than professional phagocytic cells. They also show that serum proteins other than complement components mediate the removal of apoptotic cells by murine mesangial cells in vitro.
Figures
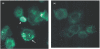

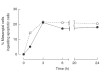


 , C1q-deficient serum;
, C1q-deficient serum;  , C3-deficient serum; □, no serum.
, C3-deficient serum; □, no serum.

References
-
- Savill J, Dransfield I, Hogg N, et al. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–3. - PubMed
-
- Fadok VA, Bratton DL, Rose DM, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. - PubMed
-
- Devitt A, Moffatt OD, Raykundalia C, et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

