Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis
- PMID: 12452848
- PMCID: PMC1906557
- DOI: 10.1046/j.1365-2249.2002.02017.x
Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis
Abstract
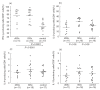
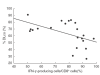
In patients with systemic sclerosis (SSc), there are conflicting findings regarding which is predominant between type 1 and type 2 immune responses. To determine the balance between type 1 and type 2 T lymphocytes in peripheral blood from SSc patients, we investigated the expression of intracellular cytokines, such as interferon-gamma (IFN-gamma), interleukin-2 (IL-2), IL-4, and IL-13, and chemokine receptors such as CXCR3 and CCR4 by flow cytometry. The frequency of IFN-gamma-producing cells among CD8+ cells was significantly increased in patients with diffuse cutaneous SSc (n = 11, P < 0.0001) and limited cutaneous SSc (lSSc; n= 16, P < 0.0001) compared with normal controls (n = 17) while there was no significant difference in the frequency of IL-4- or IL-13-producing cells. In contrast, the frequency of IFN-gamma- or IL-4-producing cells among CD4+ cells was similar between the three groups. Similar results were obtained when absolute numbers were assessed. The frequency of IFN-gamma-producing cells among CD8+ cells inversely correlated with percentage DLco in SSc patients (r = - 0.650, P < 0.005). CXCR3+ CD8+ cells selectively produced IFN-gamma, and the frequency of CXCR3+ CD45RO+ cells among CD8+ cells was higher in lSSc patients (n = 14, P < 0.01) than in normal controls (n = 22). In contrast, there was no significant difference in the frequencies of CXCR3- or CCR4-expressing CD45RO+ cells among CD4+ cells. These results demonstrate the predominance of type 1 cytokine-producing cells (Tc1 cells) in peripheral blood CD8+ T cells from SSc patients, but no definite Th1/Th2 imbalance in CD4+ T cells. Tc1 cells may be associated with pulmonary vascular damage in SSc.
Figures




References
-
- Gruschwitz M, Sepp N, Kofler H, Wick G. Expression of class II-MHC antigens in the dermis of patients with progressive systemic sclerosis. Immunobiology. 1991;182:234–55. - PubMed
-
- Gruschwitz M, von den Vieth G. Up-regulation of class II major histocompatibility complex and intercellular adhesion molecule 1 expression on scleroderma fibroblasts and endothelial cells by interferon-gamma and tumor necrosis factor alpha in the early disease stage. Arthritis Rheum. 1997;40:540–50. - PubMed
-
- Gruschwitz M, von den Driesch P, Kellner I, Hornstein OP, Sterry W. Expression of adhesion proteins involved in cell-cell and cell–matrix interactions in the skin of patients with progressive systemic sclerosis. J Am Acad Dermatol. 1992;27:169–77. - PubMed
-
- Sollberg S, Peltonen J, Uitto J, Jimenez SA. Elevated expression of beta 1 and beta 2 integrins, intercellular adhesion molecule 1, and endothelial leukocyte adhesion molecule 1 in the skin of patients with systemic sclerosis of recent onset. Arthritis Rheum. 1992;35:290–8. - PubMed
-
- Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

