Calibrated real-time PCR assay for quantitation of human herpesvirus 8 DNA in biological fluids
- PMID: 12454167
- PMCID: PMC154587
- DOI: 10.1128/JCM.40.12.4652-4658.2002
Calibrated real-time PCR assay for quantitation of human herpesvirus 8 DNA in biological fluids
Abstract
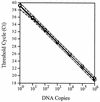
Accurate laboratory tests for the diagnosis of active human herpesvirus 8 (HHV-8) infection are becoming essential to study the pathogenesis of HHV-8-associated tumors and for the clinical management of HHV-8-infected individuals. We have developed a highly sensitive, calibrated quantitative real-time PCR assay for the measurement of cell-free HHV-8 DNA in body fluids, based on the addition of a synthetic DNA calibrator prior to DNA extraction. The calibrator controls each sample for the presence of PCR inhibitors, determines a cutoff value of sensitivity for negative samples, and normalizes positive samples for the efficiency of DNA recovery. The assay shows a wide dynamic range of detection (between 1 and 10(6) viral genome equivalents/reaction) and a high degree of accuracy even in the presence of high amounts (up to 1 micro g) of human genomic DNA. Moreover, the assay has a very high sensitivity (lower detection limit, 10 genome equivalents/ml) and a high degree of reproducibility and repeatability with a coefficient of variation (CV) of <15 and 23%, respectively. Furthermore, the use of the calibrator improves the accuracy of quantitation and decreases the intersample variability (CV, 9 and 6%, respectively). The sensitivity and specificity of the assay were tested with a series of clinical specimens obtained from patients affected by various HHV-8-related diseases, as well as from a wide number of controls. In conclusion, our calibrated real-time PCR assay provides a reliable high-throughput method for quantitation of HHV-8 DNA in clinical and laboratory specimens.
Figures

References
-
- Biggar, R. J., D. Whitby, V. Marshall, A. C. Linhares, and F. Black. 2000. Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype. J. Infect. Dis. 181:1562-1568. - PubMed
-
- Boivin, G., A. Gaudreau, and J. P. Routy. 2000. Evaluation of the human herpesvirus 8 DNA load in blood and Kaposi's sarcoma skin lesions from AIDS patients on highly active antiretroviral therapy. AIDS 14:1907-1910. - PubMed
-
- Boivin, G., A. Gaudreau, E. Toma, R. Lalonde, J. P. Routy, G. Murray, J. Handfield, and M. G. Bergeron. 1999. Human herpesvirus 8 DNA load in leukocytes of human immunodeficiency virus-infected subjects: correlation with the presence of Kaposi's sarcoma and response to anticytomegalovirus therapy. Antimicrob. Agents Chemother. 43:377-380. - PMC - PubMed
-
- Calabro, M. L., J. Sheldon, A. Favero, G. R. Simpson, J. R. Fiore, E. Gomes, G. Angarano, L. Chieco-Bianchi, and T. F. Schulz. 1998. Seroprevalence of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J. Hum. Virol. 1:207-213. - PubMed
-
- Campbell, T. B., M. Borok, L. Gwanzura, S. MaWhinney, I. E. White, B. Ndemera, I. Gudza, L. Fitzpatrick, and R. T. Schooley. 2000. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS 14:2109-2116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

