Development of a real-time fluorescence PCR assay for rapid detection of the diphtheria toxin gene
- PMID: 12454177
- PMCID: PMC154649
- DOI: 10.1128/JCM.40.12.4713-4719.2002
Development of a real-time fluorescence PCR assay for rapid detection of the diphtheria toxin gene
Abstract
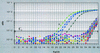
We developed and evaluated a real-time fluorescence PCR assay for detecting the A and B subunits of diphtheria toxin (tox) gene. When 23 toxigenic Corynebacterium diphtheriae strains, 9 nontoxigenic C. diphtheriae strains, and 44 strains representing the diversity of pathogens and normal respiratory flora were tested, this real-time PCR assay exhibited 100% sensitivity and specificity. It allowed for the detection of both subunits of the tox gene at 750 times greater sensitivity (2 CFU) than the standard PCR (1,500 CFU). When used directly on specimens collected from patients with clinical diphtheria, one or both subunits of the tox gene were detected in 34 of 36 specimens by using the real-time PCR assay; only 9 specimens were found to be positive by standard PCR. Reamplification by standard PCR and DNA sequencing of the amplification product confirmed all real-time PCR tox-positive reactions. This real-time PCR format is a more sensitive and rapid alternative to standard PCR for detection of the tox gene in clinical material.
Figures


References
-
- Dittmann, S., M. Wharton, C. Vitek, M. Ciotti, A. Galazka, S. Guichard, I. Hardy, U. Kartoglu, S. Koyama, J. Kreysler, B. Martin, D. Mercer, T. Ronne, C. Roure, R. Steinglass, P. Strebel, R. Sutter, and M. Trostle. 2000. Successful control of epidemic diphtheria in the states of the Former Union of Soviet Socialist Republics: lessons learned. J. Infect. Dis. 181(Suppl. 1):S10-S22. - PubMed
-
- Efstratiou, A., and P. A. Maple. 1994. WHO manual for the laboratory diagnosis of diptheria, p. 69-71. Document ICP-EPI 038(C). World Health Organization, Geneva, Switzerland.
-
- Funke, G., and K. A. Berhard. 1999. Coryneform gram-positive rods, p. 319-345. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
-
- Henricson, B., M. Segarra, J. Garvin, J. Burns, S. Jankins, C. Kim, T. Popovic, A. Golaz, and B. Akey. 2000. Toxigenic Corynebacterium diphtheriae associated with an equine wound infection. J. Vet. Diagn. Investig. 12:253-257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

