Disulfide bond-mediated dimerization of HLA-G on the cell surface
- PMID: 12454284
- PMCID: PMC138585
- DOI: 10.1073/pnas.212643199
Disulfide bond-mediated dimerization of HLA-G on the cell surface
Abstract
HLA-G is a nonclassical class I MHC molecule with an unknown function and with unusual characteristics that distinguish it from other class I MHC molecules. Here, we demonstrate that HLA-G forms disulfide-linked dimers that are present on the cell surface. Immunoprecipitation of HLA-G from surface biotinylated transfectants using the anti-beta2-microglobulin mAb BBM.1 revealed the presence of an approximately equal 78-kDa form of HLA-G heavy chain that was reduced by using DTT to a 39-kDa form. Mutation of Cys-42 to a serine completely abrogated dimerization of HLA-G, suggesting that the disulfide linkage formed exclusively through this residue. A possible interaction between the HLA-G monomer or dimer and the KIR2DL4 receptor was also investigated, but no interaction between these molecules could be detected through several approaches. The cell-surface expression of dimerized HLA-G molecules may have implications for HLA-Greceptor interactions and for the search for specific receptors that bind HLA-G.
Figures


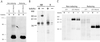



References
-
- Kovats S., Main, E. K., Librach, C., Stubblebine, M., Fisher, S. J. & DeMars, R. (1990) Science 248, 220-223. - PubMed
-
- McMaster M. T., Librach, C. L., Zhou, Y., Lim, K. H., Janatpour, M. J., DeMars, R., Kovats, S., Damsky, C. & Fisher, S. J. (1995) J. Immunol. 154, 3771-3778. - PubMed
-
- Alizadeh M., Legras, C., Semana, G., Le Bouteiller, P., Genetet, B. & Fauchet, R. (1993) Hum. Immunol. 38, 206-212. - PubMed
-
- Kirszenbaum M., Djoulah, S., Hors, J., Le Gall, I., de Oliveira, E. B., Prost, S., Dausset, J. & Carosella, E. D. (1997) Hum. Immunol. 53, 140-147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

