Liver regeneration is an angiogenesis- associated phenomenon
- PMID: 12454508
- PMCID: PMC1422636
- DOI: 10.1097/00000658-200212000-00002
Liver regeneration is an angiogenesis- associated phenomenon
Abstract
Objective: To investigate whether liver regeneration is an angiogenesis-associated phenomenon.
Summary background data: Angiogenesis is predominantly known for its pivotal role in tumor growth. However, angiogenesis could also play a role in physiologic processes involving tissue repair, such as liver regeneration.
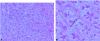
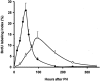
Methods: Mice subjected to 70% partial hepatectomy were treated with human angiostatin (100 mg/kg body weight). Regeneration-induced hepatic angiogenesis was determined by assessing intrahepatic microvascular density using CD31 staining of frozen liver sections. Liver regeneration was evaluated by assessing wet liver weights and BrdU incorporation in DNA at regular intervals after partial hepatectomy. Possible direct effects of angiostatin on hepatocytes were studied by assessment of liver enzymes (ASAT, ALAT, bilirubin, lactate dehydrogenase), MTT assay (cytotoxicity), aminophenol production (metabolic function), and TUNEL (apoptosis).
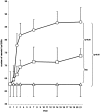
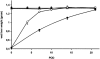
Results: In a regenerating liver, microvascular density increased by 38%. Angiostatin significantly inhibited this response by 60%. In addition, angiostatin inhibited liver regeneration by 50.4% and 24.9% on postoperative days 7 and 14, respectively. In control mice liver weights regained normalcy in 8 days, whereas those in angiostatin-treated mice normalized after 21 days. In angiostatin-treated mice, the maximal BrdU incorporation was decreased and delayed. Direct adverse effects of angiostatin on cultured and in vivo hepatocytes were not observed. Angiostatin neither induced necrosis on hematoxylin and eosin staining nor affected serum levels of liver enzymes.
Conclusions: Liver regeneration is accompanied by intrahepatic angiogenesis. Antiangiogenic treatment using angiostatin inhibits both phenomena. The authors conclude that liver regeneration is, at least in part, an angiogenesis-dependent phenomenon.
Figures


References
-
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71. - PubMed
-
- Kerbel RS. Clinical trials of antiangiogenic drugs: opportunities, problems, and assessment of initial results. J Clin Oncol 2001; 19: 45S–51S. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. - PubMed
-
- Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995; 1: 149–153. - PubMed
-
- Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann NY Acad Sci 1992; 667: 101–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

