A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers
- PMID: 12454761
- PMCID: PMC2376292
- DOI: 10.1038/sj.bjc.6600644
A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers
Abstract
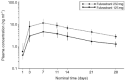
While tamoxifen use is associated with clear benefits in the treatment of hormone-sensitive breast cancer, it also exhibits partial oestrogen agonist activity that is associated with adverse events, including endometrial cancer. Fulvestrant ("Faslodex") is a new oestrogen receptor antagonist that downregulates the oestrogen receptor and has no known agonist effect. This single-centre, double-blind, randomised, parallel-group trial was conducted to determine the direct effects of fulvestrant on the female endometrium when given alone and in combination with the oestrogen, ethinyloestradiol. Following a 14-day, pretrial screening period, 30 eligible postmenopausal volunteers were randomised to receive fulvestrant 250 mg, fulvestrant 125 mg or matched placebo administered as a single intramuscular injection. Two weeks postinjection, volunteers received 2-weeks concurrent exposure to ethinyloestradiol 20 microg day(-1). Endometrial thickness was measured before and after the 14-day screening period with further measurements predose (to confirm a return to baseline) and on days 14, 28 and 42 post-treatment with fulvestrant. Pharmacokinetic and safety assessments were performed throughout the trial. Fulvestrant at a dose of 250 mg significantly (P=0.0001) inhibited the oestrogen-stimulated thickening of the endometrium compared with placebo. Neither the 125 mg nor 250 mg doses of fulvestrant demonstrated oestrogenic effects on the endometrium over the initial 14-day assessment period. Fulvestrant was well tolerated and reduced the incidence of ethinyloestradiol-related side effects. At the same dose level that is being evaluated in clinical trials of postmenopausal women with advanced breast cancer, fulvestrant (250 mg) is an antioestrogen with no evidence of agonist activity in the endometrium of healthy postmenopausal women.
Copyright 2002 Cancer Research UK
Figures

References
-
- BergmanLBeelenMLGalleeMPHollemaHBenraadtJvan LeeuwenFE2000Risk and prognosis of endometrial cancer after tamoxifen for breast cancer Lancet 356881887 - PubMed
-
- BilmoriaMMJordanVCMorrowM1996Additional benefits of tamoxifen for postmenopausal patientsInTamoxifen a guide for clinicians and patientsJordan VC (ed)pp 75–89Huntington NY: PRR Inc
-
- ChlebowskiRCollyarDESomerfieldMRPfisterDG1999American Society of Clinical Oncology Technology. Assessment of breast cancer risk reduction strategies: tamoxifen and raloxifene J Clin Oncol 1719391955 - PubMed
-
- DeFriendDJHowellANicholsonRIAndersonEDowsettMManselREBlameyRWBundredNJRobertsonJFSaundersCet al1994Investigation of a new pure antiestrogen (ICI 182780) in women with primary breast cancer Cancer Res 54408414 - PubMed
-
- DukesMMillerDWakelingAEWatertonJC1992Antiuterotrophic effects of a pure antioestrogen, ICI 182780; magnetic resonance imaging of the uterus in ovariectomised monkeys J Endocrinol 135239247 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

