Block sequential adriamycin CMF--optimal non-myeloablative chemotherapy for high risk adjuvant breast cancer?
- PMID: 12454763
- PMCID: PMC2376297
- DOI: 10.1038/sj.bjc.6600660
Block sequential adriamycin CMF--optimal non-myeloablative chemotherapy for high risk adjuvant breast cancer?
Abstract
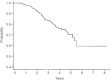
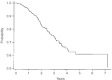
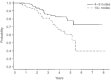
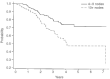
After the publication of the 10-year survival data from Milan on the adjuvant use of the block sequential regimen consisting of four cycles of adriamycin followed by eight cycles of intravenous CMF, many centres adopted this as standard of care for high risk, multiple node-positive breast cancer. For this reason it was identified as the standard arm for the Anglo-Celtic adjuvant high-dose chemotherapy trial. This study reports on the experience of this regimen in 329 women with early breast cancer involving at least four axillary nodes, who were treated outside any adjuvant chemotherapy trial. At a median follow-up of 3 years, the overall 5-year disease-free survival is 61%, and the overall survival is 70%. These data confirm the efficacy of this regimen in non-trial patients, and, for the same high risk subgroup, indicate that this approach offers an outcome at least as good as that seen in the CALGB 9344 AC-Taxol arm, and the NCIC days 1 and 8 CEF.
Copyright 2002 Cancer Research UK
Figures
References
-
- BerghJWiklundTEriksteinBLidbrinkELindmanHMalmstromPKellokumpu-LehtinenPBengtsson NOSoderlundGAnkerGWistEOttossonSSalminenELjungmanPHolteHNilssonJBlomqvistCWilkingN2000Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: a randomised trial. Scandinavian Breast Group 9401 study Lancet 35613841391 - PubMed
-
- BonadonnaGZambettiMValagussaP1995Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results[see comments]JAMA 273542547 - PubMed
-
- BuzzoniRBonadonnaGValagussaPZambettiM1991Adjuvant chemotherapy with doxorubicin plus cyclophosphamide, methotrexate, and fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes J Clin Oncol 921342140 - PubMed
-
- Early Breast Cancer Trialists Collaborative Group1998Polychemotherapy for early breast cancer: an overview of the randomised trials Lancet 352930942 - PubMed
-
- Early Breast Cancer Trialists Collaborative Group2000. Unpublished Overview data for polychemotherapy for early breast cancer - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical