Copper-dependent iron assimilation pathway in the model photosynthetic eukaryote Chlamydomonas reinhardtii
- PMID: 12455693
- PMCID: PMC126744
- DOI: 10.1128/EC.1.5.736-757.2002
Copper-dependent iron assimilation pathway in the model photosynthetic eukaryote Chlamydomonas reinhardtii
Abstract
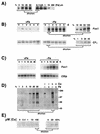
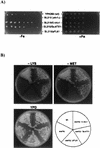
The unicellular green alga Chlamydomonas reinhardtii is a valuable model for studying metal metabolism in a photosynthetic background. A search of the Chlamydomonas expressed sequence tag database led to the identification of several components that form a copper-dependent iron assimilation pathway related to the high-affinity iron uptake pathway defined originally for Saccharomyces cerevisiae. They include a multicopper ferroxidase (encoded by Fox1), an iron permease (encoded by Ftr1), a copper chaperone (encoded byAtx1), and a copper-transporting ATPase. A cDNA, Fer1, encoding ferritin for iron storage also was identified. Expression analysis demonstrated that Fox1 and Ftrl were coordinately induced by iron deficiency, as were Atx1 and Fer1, although to lesser extents. In addition, Fox1 abundance was regulated at the posttranscriptional level by copper availability. Each component exhibited sequence relationship with its yeast, mammalian, or plant counterparts to various degrees; Atx1 of C. reinhardtii is also functionally related with respect to copper chaperone and antioxidant activities. Fox1 is most highly related to the mammalian homologues hephaestin and ceruloplasmin; its occurrence and pattern of expression in Chlamydomonas indicate, for the first time, a role for copper in iron assimilation in a photosynthetic species. Nevertheless, growth of C. reinhardtii under copper- and iron-limiting conditions showed that, unlike the situation in yeast and mammals, where copper deficiency results in a secondary iron deficiency, copper-deficient Chlamydomonas cells do not exhibit symptoms of iron deficiency. We propose the existence of a copper-independent iron assimilation pathway in this organism.
Figures










References
-
- Abboud, S., and D. J. Haile. 2000. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275:19906-19912. - PubMed
-
- Aisen, P., M. Wessling-Resnick, and E. A. Leibold. 1999. Iron metabolism. Curr. Opin. Chem. Biol. 3:200-206. - PubMed
-
- Andrews, N. C. 1999. The iron transporter DMT1. Int. J. Biochem. Cell Biol. 31:991-994. - PubMed
-
- Asamizu, E., Y. Nakamura, S. Sato, H. Fukuzawa, and S. Tabata. 1999. A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res. 6:369-373. - PubMed
-
- Askwith, C., D. Eide, A. Van Ho, P. S. Bernard, L. Li, S. Davis-Kaplan, D. M. Sipe, and J. Kaplan. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403-410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

