Aspergillus nidulans swoF encodes an N-myristoyl transferase
- PMID: 12455958
- PMCID: PMC118038
- DOI: 10.1128/EC.1.2.241-248.2002
Aspergillus nidulans swoF encodes an N-myristoyl transferase
Abstract
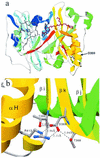
Polar growth is a fundamental process in filamentous fungi and is necessary for disease initiation in many pathogenic systems. Previously, swoF was identified in Aspergillus nidulans as a single-locus, temperature-sensitive (ts) mutant aberrant in both polarity establishment and polarity maintenance. The swoF gene was cloned by complementation of the ts phenotype and sequenced. The derived protein sequence had high identity with N-myristoyl transferases (NMTs) found in fungi, plants, and animals. In addition, wild-type growth at restrictive temperature was partially restored by the addition of myristic acid to the growth medium. Sequencing revealed that the mutation in swoF changes the conserved aspartic acid 369 to a tyrosine. The predicted A. nidulans SwoF protein, SwoFp, was homology modeled based on crystal structures of NMTs from Saccharomyces cerevisiae and Candida albicans. The D369Y swoF mutation is on the opposite face of the protein, distal to the myristoyl coenzyme A and peptide substrate binding sites. In wild-type NMTs, D369 appears to stabilize a structural beta-strand bend through two hydrogen bonds and an ionic interaction. These stabilizing bonds are abolished in the D369Y mutant. We hypothesize that a substrate of SwoFp must be myristoylated for proper polarity establishment and maintenance. The mutation prevents the proper function of SwoFp at restrictive temperature and thus blocks polar growth.
Figures




Similar articles
-
SwoHp, a nucleoside diphosphate kinase, is essential in Aspergillus nidulans.Eukaryot Cell. 2003 Dec;2(6):1169-77. doi: 10.1128/EC.2.6.1169-1177.2003. Eukaryot Cell. 2003. PMID: 14665452 Free PMC article.
-
Comparison of myristoyl-CoA:protein N-myristoyltransferases from three pathogenic fungi: Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans.J Biol Chem. 1994 Jan 28;269(4):2996-3009. J Biol Chem. 1994. PMID: 8300631
-
Genetic and biochemical studies of a mutant Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase, nmt72pLeu99-->Pro, that produces temperature-sensitive myristic acid auxotrophy.J Biol Chem. 1993 Jan 5;268(1):483-94. J Biol Chem. 1993. PMID: 8416952
-
Myristic acid auxotrophy caused by mutation of S. cerevisiae myristoyl-CoA:protein N-myristoyltransferase.J Cell Biol. 1991 Jun;113(6):1313-30. doi: 10.1083/jcb.113.6.1313. J Cell Biol. 1991. PMID: 2045414 Free PMC article.
-
The Aspergillus nidulans lysF gene encodes homoaconitase, an enzyme involved in the fungus-specific lysine biosynthesis pathway.Mol Gen Genet. 1997 Jul;255(3):237-47. doi: 10.1007/s004380050494. Mol Gen Genet. 1997. PMID: 9268014
Cited by
-
Impaired ribosome biogenesis disrupts the integration between morphogenesis and nuclear duplication during the germination of Aspergillus fumigatus.Eukaryot Cell. 2008 Apr;7(4):575-83. doi: 10.1128/EC.00412-07. Epub 2008 Feb 22. Eukaryot Cell. 2008. PMID: 18296619 Free PMC article.
-
SwoHp, a nucleoside diphosphate kinase, is essential in Aspergillus nidulans.Eukaryot Cell. 2003 Dec;2(6):1169-77. doi: 10.1128/EC.2.6.1169-1177.2003. Eukaryot Cell. 2003. PMID: 14665452 Free PMC article.
-
Protein myristoylation in health and disease.J Chem Biol. 2010 Mar;3(1):19-35. doi: 10.1007/s12154-009-0032-8. Epub 2009 Nov 7. J Chem Biol. 2010. PMID: 19898886 Free PMC article.
-
N-myristoyltransferase is a cell wall target in Aspergillus fumigatus.ACS Chem Biol. 2015 Jun 19;10(6):1425-34. doi: 10.1021/cb5008647. Epub 2015 Feb 27. ACS Chem Biol. 2015. PMID: 25706802 Free PMC article.
-
Ras GTPase-activating protein regulation of actin cytoskeleton and hyphal polarity in Aspergillus nidulans.Eukaryot Cell. 2008 Jan;7(1):141-53. doi: 10.1128/EC.00346-07. Epub 2007 Nov 26. Eukaryot Cell. 2008. PMID: 18039943 Free PMC article.
References
-
- Aleksenko, A., and A. J. Clutterbuck. 1997. Autonomous plasmid replication in Aspergillus nidulans: AMA1 and MATE elements. Fungal Genet. Biol. 21:373-387. - PubMed
-
- Ballance, D. J. 1986. Sequences important for gene expression in filamentous fungi. Yeast 2:229-236. - PubMed
-
- Bhatnagar, R. S., K. Futterer, T. A. Farazi, S. Korolev, C. L. Murray, E. Jackson-Machelski, G. W. Gokel, J. I. Gordon, and G. Waksman. 1998. Structure of N-myristoyltransferase with bound myristoyl-CoA and peptide substrate analogs. Nat. Struct. Biol. 5:1091-1097. - PubMed
-
- Bhatnagar, R. S., K. Futterer, G. Waksman, and J. I. Gordon. 1999. The structure of myristoyl-CoA: protein N-myristoyltransferase. Biochim. Biophys. Acta 1441:162-172. - PubMed
-
- Bhatnagar, R. S., and J. I. Gordon. 1997. Understanding covalent modifications of lipids: where cell biology and biophysics mingle. Trends Cell Biol. 7:14-20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

