Roles of TUP1 in switching, phase maintenance, and phase-specific gene expression in Candida albicans
- PMID: 12455984
- PMCID: PMC118011
- DOI: 10.1128/EC.1.3.353-365.2002
Roles of TUP1 in switching, phase maintenance, and phase-specific gene expression in Candida albicans
Abstract
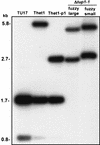
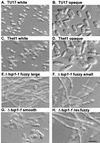
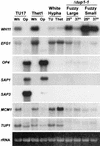
Candida albicans strain WO-1 switches spontaneously and reversibly between a "white" and "opaque" phenotype that affects colony morphology, cellular phenotype, and expression of a number of phase-specific genes and virulence traits. To assess the role of the transcription regulator Tup1p in this phenotypic transition, both TUP1 alleles were deleted in the mutant delta tup1. Delta tup1 formed "fuzzy large" colonies made up of cells growing exclusively in the filamentous form. Delta tup1 cells did not undergo the white-opaque transition, but it did switch spontaneously, at high frequency (approximately 10(-3)), and unidirectionally through the following sequence of colony (and cellular) phenotypes: "fuzzy large" (primarily hyphae) --> "fuzzy small" (primarily pseudohyphae) --> "smooth" (primarily budding yeast) --> "revertant fuzzy" (primarily pseudohyphae). Northern analysis of white-phase, opaque-phase, and hypha-associated genes demonstrated that Tup1p also plays a role in the regulation of select phase-specific genes and that each variant in the delta tup1 switching lineage differs in the level of expression of one or more phase-specific and/or hypha-associated genes. Using a rescued delta tup1 strain, in which TUP1 was placed under the regulation of the inducible MET3 promoter, white- and opaque-phase cells were individually subjected to a regime in which TUP1 was first downregulated and then upregulated. The results of this experiment demonstrated that (i) downregulation of TUP1 led to exclusive filamentous growth in both originally white- and opaque-phase cells; (ii) the white-phase-specific gene WH11 continued to be expressed in TUP1 downregulated cultures originating from white-phase cells, while WH11 expression remained repressed in TUP1-downregulated cultures originating from opaque-phase cells, suggesting that cells maintained phase identity in the absence of TUP1 expression; and (iii) subsequent upregulation of TUP1 resulted in mass conversion of originally white-phase cells to the opaque phase and maintenance of originally opaque-phase cells in the opaque phase and in the resumption in both cases of switching, suggesting that TUP1 reexpression turns on the switching system in the opaque phase.
Figures




References
-
- Bone, J. R., and S. Y. Roth. 2001. Recruitment of the yeast Tup1p-Ssn6p repressor is associated with localized decreases in histone deacetylation. J. Biol. Chem. 276:1808-1813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

