G-protein signaling mediates asexual development at 25 degrees C but has no effect on yeast-like growth at 37 degrees C in the dimorphic fungus Penicillium mameffei
- PMID: 12455992
- PMCID: PMC118015
- DOI: 10.1128/EC.1.3.440-447.2002
G-protein signaling mediates asexual development at 25 degrees C but has no effect on yeast-like growth at 37 degrees C in the dimorphic fungus Penicillium mameffei
Abstract
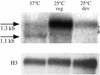
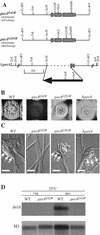
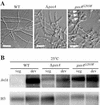
The ascomycete Penicillium marneffei is an opportunistic human pathogen exhibiting a temperature-dependent dimorphic switch. At 25 degrees C, P. marneffei grows as filamentous multinucleate hyphae and undergoes asexual development, producing uninucleate spores. At 37 degrees C, it forms uninucleate yeast cells which divide by fission. We have cloned a gene encoding a G alpha subunit of a heterotrimeric G protein from P. marneffei named gasA with high similarity to fadA in Aspergillus nidulans. Through the characterization of a delta gasA strain and mutants carrying a dominant activating or a dominant interfering gasA allele, we show that GasA is a key regulator of asexual development but seems to play no role in the regulation of growth. A dominant activating gasA mutant whose mutation results in a G42-to-R change (gasA(G42R)) does not express brlA, the conidiation-specific regulatory gene, and is locked in vegetative growth, while a dominant interfering gasA(G203R) mutant shows inappropriate brlA expression and conidiation. Interestingly, the gasA mutants have no apparent defect in dimorphic switching or yeast-like growth at 37 degrees C. Growth tests on dibutyryl cyclic AMP (dbcAMP) and theophylline suggest that a cAMP-protein kinase A cascade may be involved in the GasA signaling pathway.
Figures


References
-
- Adams, T. H., M. T. Boylan, and W. E. Timberlake. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353-362. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, et al. (ed.). 1994. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
-
- Bolker, M. 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25:143-156. - PubMed
-
- Borneman, A. R., M. J. Hynes, and A. Andrianopoulos. 2000. The abaA homologue of Penicillium marneffei participates in two developmental programmes: conidiation and dimorphic growth. Mol. Microbiol. 38:1034-1047. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

