FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth
- PMID: 12456636
- PMCID: PMC136935
- DOI: 10.1093/emboj/cdf631
FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth
Abstract
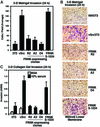
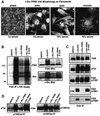
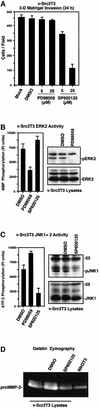
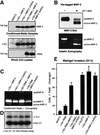
Focal adhesion kinase (FAK) was first identified as a viral Src (v-Src) substrate, but the role of FAK in Src transformation events remains undefined. We show that stable expression of the FAK C-terminal domain (termed FRNK) in v-Src-transformed NIH 3T3 fibroblasts inhibited cell invasion through Matrigel and blocked experimental metastases in nude mice without effects on cell motility. FRNK inhibitory activity was dependent upon its focal contact localization. FRNK expression disrupted the formation of a v-Src-FAK signaling complex, inhibited p130Cas tyrosine phosphorylation, and attenuated v-Src-stimulated ERK and JNK kinase activation. However, FRNK did not affect v-Src-stimulated Akt activation, cell growth in soft agar, or subcutaneous tumor formation in nude mice. FRNK-expressing cells exhibited decreased matrix metalloproteinase-2 (MMP-2) mRNA levels and MMP-2 secretion. Transient FRNK expression in human 293 cells inhibited exogenous MMP-2 promoter activity and overexpression of wild-type but not catalytically-inactive (Ala-404) MMP-2 rescued v-Src-stimulated Matrigel invasion in the presence of FRNK. Our findings show the importance of FAK in Src-stimulated cell invasion and support a role for Src-FAK signaling associated with elevated tumor cell metastases.
Figures





References
-
- Aguirre-Ghiso J.A. (2002) Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene, 21, 2513–2524. - PubMed
-
- Aguirre-Ghiso J.A., Frankel,P., Farias,E.F., Lu,Z., Jiang,H., Olsen,A., Feig,L.A., de Kier Joffe,E.B. and Foster,D.A. (1999) RalA requirement for v-Src- and v-Ras-induced tumorigenicity and overproduction of urokinase-type plasminogen activator: involvement of metallo proteases. Oncogene, 18, 4718–4725. - PubMed
-
- Behrens J., Vakaet,L., Friis,R., Winterhager,E., Van Roy,F., Mareel,M.M. and Birchmeier,W. (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol., 120, 757–766. - PMC - PubMed
-
- Boyer B., Bourgeois,Y. and Poupon,M.F. (2002) Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene, 21, 2347–2356. - PubMed
-
- Calalb M.B., Zhang,X., Polte,T.R. and Hanks,S.K. (1996) Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun., 228, 662–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

