Oncostatin M regulates membrane traffic and stimulates bile canalicular membrane biogenesis in HepG2 cells
- PMID: 12456648
- PMCID: PMC136933
- DOI: 10.1093/emboj/cdf629
Oncostatin M regulates membrane traffic and stimulates bile canalicular membrane biogenesis in HepG2 cells
Abstract
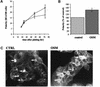
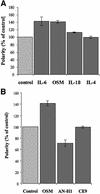
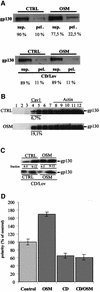
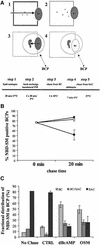
Hepatocytes are the major epithelial cells of the liver and they display membrane polarity: the sinusoidal membrane representing the basolateral surface, while the bile canalicular membrane is typical of the apical membrane. In polarized HepG2 cells an endosomal organelle, SAC, fulfills a prominent role in the biogenesis of the canalicular membrane, reflected by its ability to sort and redistribute apical and basolateral sphingolipids. Here we show that SAC appears to be a crucial target for a cytokine-induced signal transduction pathway, which stimulates membrane transport exiting from this compartment promoting apical membrane biogenesis. Thus, oncostatin M, an IL-6-type cytokine, stimulates membrane polarity development in HepG2 cells via the gp130 receptor unit, which activates a protein kinase A-dependent and sphingomyelin-marked membrane transport pathway from SAC to the apical membrane. To exert its signal transducing function, gp130 is recruited into detergent-resistant membrane microdomains at the basolateral membrane. These data provide a clue for a molecular mechanism that couples the biogenesis of an apical plasma membrane domain to the regulation of intracellular transport in response to an extracellular, basolaterally localized stimulus.
Figures



Similar articles
-
Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide.Mol Biol Cell. 2006 Aug;17(8):3638-50. doi: 10.1091/mbc.e06-03-0230. Epub 2006 May 24. Mol Biol Cell. 2006. PMID: 16723498 Free PMC article.
-
Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells.Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):15087-92. doi: 10.1073/pnas.0503702102. Epub 2005 Oct 7. Proc Natl Acad Sci U S A. 2005. PMID: 16214890 Free PMC article.
-
Anchoring of protein kinase A-regulatory subunit IIalpha to subapically positioned centrosomes mediates apical bile canalicular lumen development in response to oncostatin M but not cAMP.Mol Biol Cell. 2007 Jul;18(7):2745-54. doi: 10.1091/mbc.e06-08-0732. Epub 2007 May 9. Mol Biol Cell. 2007. PMID: 17494870 Free PMC article.
-
Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells.Biochem J. 1998 Dec 1;336 ( Pt 2)(Pt 2):257-69. doi: 10.1042/bj3360257. Biochem J. 1998. PMID: 9820799 Free PMC article. Review.
-
Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters.Semin Liver Dis. 2000;20(3):339-51. doi: 10.1055/s-2000-9388. Semin Liver Dis. 2000. PMID: 11076400 Review.
Cited by
-
Quantitative Proteomics Reveals the Roles of Peroxisome-associated Proteins in Antiviral Innate Immune Responses.Mol Cell Proteomics. 2015 Sep;14(9):2535-49. doi: 10.1074/mcp.M115.048413. Epub 2015 Jun 29. Mol Cell Proteomics. 2015. PMID: 26124285 Free PMC article.
-
NF-κB mediates the induction of Fas receptor and Fas ligand by microcystin-LR in HepG2 cells.Mol Cell Biochem. 2011 Jun;352(1-2):209-19. doi: 10.1007/s11010-011-0756-y. Epub 2011 Feb 26. Mol Cell Biochem. 2011. PMID: 21359529
-
Formation of E-cadherin/beta-catenin-based adherens junctions in hepatocytes requires serine-10 in p27(Kip1).Mol Biol Cell. 2008 Apr;19(4):1605-13. doi: 10.1091/mbc.e07-07-0661. Epub 2008 Feb 13. Mol Biol Cell. 2008. PMID: 18272788 Free PMC article.
-
Rho kinase, myosin-II, and p42/44 MAPK control extracellular matrix-mediated apical bile canalicular lumen morphogenesis in HepG2 cells.Mol Biol Cell. 2006 Jul;17(7):3291-303. doi: 10.1091/mbc.e06-01-0067. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687572 Free PMC article.
-
Induction of Bile Canaliculi-Forming Hepatocytes from Human Pluripotent Stem Cells.Methods Mol Biol. 2022;2544:71-82. doi: 10.1007/978-1-0716-2557-6_4. Methods Mol Biol. 2022. PMID: 36125710
References
-
- Babia T., Kok,J.W. and Hoekstra,D. (1994) The use of fluorescent lipid analogues to study endocytosis of glycosphingolipids. In Greenstein,B. (ed.), Receptor Research Methods. Harward Academic Publishers, London, UK, pp. 155–174.
-
- Barasch J. et al. (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell, 99, 377–386. - PubMed
-
- Brignoni M., Pignataro,O.P., Rodriguez,M.L., Alvarez,A., Vega-Salas,D.E., Rodriguez-Boulan,E. and Salas,P.J. (1995) Cyclic AMP modulates the rate of ‘constitutive’ exocytosis of apical membrane proteins in Madin–Darby canine kidney cells. J. Cell Sci., 108, 1931–1943. - PubMed
-
- Bruce A.G., Hoggatt,I.H. and Rose,T.M. (1992) Oncostatin M is a differentiation factor for myeloid leukemia cells. J. Immunol., 149, 1271–1275. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

