XPak3 promotes cell cycle withdrawal during primary neurogenesis in Xenopus laevis
- PMID: 12456650
- PMCID: PMC136948
- DOI: 10.1093/emboj/cdf644
XPak3 promotes cell cycle withdrawal during primary neurogenesis in Xenopus laevis
Abstract
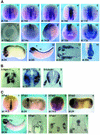
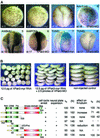
We have isolated the Xenopus p21-activated kinase 3 (XPak3) by virtue of its expression in the territory of primary neurogenesis in the developing embryo. XPak3, but not the other Pak variants, responds positively to X-Ngnr-1 and negatively to X-Notch-1. A constitutively active form of XPak3, generated by fusing a myristylation signal to the N-terminus (XPak3-myr), induces early cell cycle arrest at high concentrations, while ectopic expression of low amounts induces premature neuronal differentiation. Conversely, XPak3 loss of function achieved by use of an antisense morpholino oligonucleotide increases cell proliferation and inhibits neuronal differentiation; this phenotype is rescued by co-injection of XPak3-myr. We conclude that XPak3 is a novel member of the proneural pathway, functioning downstream of neurogenin to withdraw neuronally programmed cells from the mitotic cell cycle, thus allowing for their differentiation.
Figures



Similar articles
-
Mxi1 is essential for neurogenesis in Xenopus and acts by bridging the pan-neural and proneural genes.Dev Biol. 2006 Apr 15;292(2):470-85. doi: 10.1016/j.ydbio.2005.12.037. Epub 2006 Feb 2. Dev Biol. 2006. PMID: 16457797
-
Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate.Development. 2003 Nov;130(21):5143-54. doi: 10.1242/dev.00665. Development. 2003. PMID: 12975341
-
Comparative expression analysis of the neurogenins in Xenopus tropicalis and Xenopus laevis.Dev Dyn. 2009 Feb;238(2):451-8. doi: 10.1002/dvdy.21845. Dev Dyn. 2009. PMID: 19161242
-
Coordination of cell cycle exit and differentiation of neuronal progenitors.Cell Cycle. 2008 Mar 15;7(6):691-7. doi: 10.4161/cc.7.6.5550. Epub 2008 Jan 1. Cell Cycle. 2008. PMID: 18239460 Review.
-
[Length of cell cycle in neural development].Yi Chuan. 2011 Nov;33(11):1185-90. doi: 10.3724/sp.j.1005.2011.01185. Yi Chuan. 2011. PMID: 22120073 Review. Chinese.
Cited by
-
NumbL is essential for Xenopus primary neurogenesis.BMC Dev Biol. 2013 Oct 14;13:36. doi: 10.1186/1471-213X-13-36. BMC Dev Biol. 2013. PMID: 24125469 Free PMC article.
-
The p21-activated kinases in neural cytoskeletal remodeling and related neurological disorders.Protein Cell. 2022 Jan;13(1):6-25. doi: 10.1007/s13238-020-00812-9. Epub 2020 Dec 11. Protein Cell. 2022. PMID: 33306168 Free PMC article. Review.
-
p21-activated kinase 3 is overexpressed in thymic neuroendocrine tumors (carcinoids) with ectopic ACTH syndrome and participates in cell migration.Endocrine. 2010 Aug;38(1):38-47. doi: 10.1007/s12020-010-9324-6. Epub 2010 May 4. Endocrine. 2010. PMID: 20960100
-
PAK-PIX interactions regulate adhesion dynamics and membrane protrusion to control neurite outgrowth.J Cell Sci. 2013 Mar 1;126(Pt 5):1122-33. doi: 10.1242/jcs.112607. Epub 2013 Jan 15. J Cell Sci. 2013. PMID: 23321640 Free PMC article.
-
Retinoic acid signaling and neuronal differentiation.Cell Mol Life Sci. 2015 Apr;72(8):1559-76. doi: 10.1007/s00018-014-1815-9. Epub 2015 Jan 6. Cell Mol Life Sci. 2015. PMID: 25558812 Free PMC article. Review.
References
-
- Allen C.M., Gleeson,J.G., Bagrodia,S., Partington,M.W., MacMillan,J.C., Cerione,R.A., Mulley,J.C. and Walsh,C.A. (1998) Pak3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet., 20, 25–30. - PubMed
-
- Bagrodia S. and Cerione,R.A. (1999) Pak to the future. Trends Cell Biol., 9, 350–355. - PubMed
-
- Bagrodia S., Taylor,S.J., Creasy,C.L., Chernoff,J. and Cerione,R.A. (1995) Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem., 270, 22731–22737. - PubMed
-
- Bellefroid E.J., Bourguignon,C., Holleman,T., Ma,Q., Anderson,D.J., Kintner,C. and Pieler,T. (1996) X-MyT1, a Xenopus C2HC-type zinc finger protein with a regulatory function in neuronal differentiation. Cell, 87, 1191–1202. - PubMed
-
- Bienvenu T. et al. (2000) Missense mutation in Pak3, R67C, causes X-linked nonspecific mental retardation. Am. J. Med. Genet., 93, 294–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

