BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins
- PMID: 12456653
- PMCID: PMC136961
- DOI: 10.1093/emboj/cdf658
BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins
Abstract
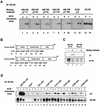
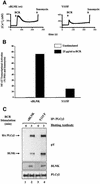
Assembly of intracellular macromolecular complexes is thought to provide an important mechanism to coordinate the generation of second messengers upon receptor activation. We have previously identified a B cell linker protein, termed BLNK, which serves such a scaffolding function in B cells. We demonstrate here that phosphorylation of five tyrosine residues within human BLNK nucleates distinct signaling effectors following B cell antigen receptor activation. The phosphorylation of multiple tyrosine residues not only amplifies PLCgamma-mediated signaling but also supports 'cis'-mediated interaction between distinct signaling effectors within a large molecular complex. These data demonstrate the importance of coordinate phosphorylation of molecular scaffolds, and provide insights into how assembly of macromolecular complexes is required for normal receptor function.
Figures






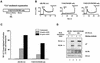
References
-
- Benschop R. and Cambier,J. (1999) B cell development: signal transduction by antigen receptors and their surrogates. Curr. Opin. Immunol., 11, 143–151. - PubMed
-
- Cheng A.M., Rowley,R.B., Pao,W., Hayday,A., Bolen,J.B. and Pawson,T. (1995) Syk tyrosine kinase required for mouse viability and B-cell development. Nature, 378, 303–306. - PubMed
-
- Costello P., Walters,A., Mee,P., Turner,M., Reynolds,L., Prisco,A., Sarner,N., Zamoyska,R. and Tybulewicz,V. (1999) The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κ B pathways. Proc. Natl Acad. Sci. USA, 96, 3035–3040. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

