Gating properties of GIRK channels activated by Galpha(o)- and Galpha(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation
- PMID: 12456817
- PMCID: PMC2290703
- DOI: 10.1113/jphysiol.2002.032151
Gating properties of GIRK channels activated by Galpha(o)- and Galpha(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation
Abstract
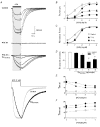
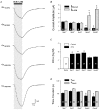
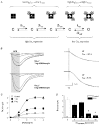
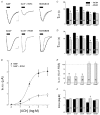
'Regulators of G protein Signalling' (RGSs) accelerate the activation and deactivation kinetics of G protein-gated inwardly rectifying K(+) (GIRK) channels. In an apparent paradox, RGSs do not reduce steady-state GIRK current amplitudes as expected from the accelerated rate of deactivation when reconstituted in Xenopus oocytes. We present evidence here that this kinetic anomaly is dependent on the degree of G protein-coupled receptor (GPCR) precoupling, which varies with different Galpha(i/o)-RGS complexes. The gating properties of GIRK channels (Kir3.1/Kir3.2a) activated by muscarinic m2 receptors at varying levels of G protein expression were examined with or without the co-expression of either RGS4 or RGS7 in Xenopus oocytes. Different levels of specific m2 receptor-Galpha coupling were established by uncoupling endogenous pertussis toxin (PTX)-sensitive Galpha(i/o) subunits with PTX, while expressing varying amounts of a single PTX-insensitive subunit (Galpha(i1(C351G)), Galpha(i2(C352G)), Galpha(i3(C351G)), Galpha(oA(C351G)), or Galpha(oB(C351G))). Co-expression of each of the PTX-insensitive Galpha(i/o) subunits rescued acetylcholine (ACh)-elicited GIRK currents (I(K,ACh)) in a concentration-dependent manner, with Galpha(o) isoforms being more effective than Galpha(i) isoforms. Receptor-independent 'basal' GIRK currents (I(K,basal)) were reduced with increasing expression of PTX-insensitive Galpha subunits and were accompanied by a parallel rise in I(K,ACh). These effects together are indicative of increased Gbetagamma scavenging by the expressed Galpha subunit and the subsequent formation of functionally coupled m2 receptor-G protein heterotrimers (Galpha((GDP))betagamma). Co-expression of RGS4 accelerated all the PTX-insensitive Galpha(i/o)-coupled GIRK currents to a similar extent, yet reduced I(K,ACh) amplitudes 60-90 % under conditions of low Galpha(i/o) coupling. Kinetic analysis indicated the RGS4-dependent reduction in steady-state GIRK current was fully explained by the accelerated deactivation rate. Thus kinetic inconsistencies associated with RGS4-accelerated GIRK currents occur at a critical threshold of G protein coupling. In contrast to RGS4, RGS7 selectively accelerated Galpha(o)-coupled GIRK currents. Co-expression of Gbeta5, in addition to enhancing the kinetic effects of RGS7, caused a significant reduction (70-85 %) in steady-state GIRK currents indicating RGS7-Gbeta5 complexes disrupt Galpha(o) coupling. Altogether these results provide further evidence for a GPCR-Galphabetagamma-GIRK signalling complex that is revealed by the modulatory affects of RGS proteins on GIRK channel gating. Our functional experiments demonstrate that the formation of this signalling complex is markedly dependent on the concentration and composition of G protein-RGS complexes.
Figures



Similar articles
-
Gbetagamma-activated inwardly rectifying K(+) (GIRK) channel activation kinetics via Galphai and Galphao-coupled receptors are determined by Galpha-specific interdomain interactions that affect GDP release rates.J Biol Chem. 2004 Jul 9;279(28):29787-96. doi: 10.1074/jbc.M403359200. Epub 2004 Apr 27. J Biol Chem. 2004. PMID: 15123672
-
Neuronal Kir3.1/Kir3.2a channels coupled to serotonin 1A and muscarinic m2 receptors are differentially modulated by the "short" RGS3 isoform.Neuropharmacology. 2005 Sep;49(4):465-76. doi: 10.1016/j.neuropharm.2005.04.010. Neuropharmacology. 2005. PMID: 15935408
-
Coupling of the muscarinic m2 receptor to G protein-activated K(+) channels via Galpha(z) and a receptor-Galpha(z) fusion protein. Fusion between the receptor and Galpha(z) eliminates catalytic (collision) coupling.J Biol Chem. 2000 Feb 11;275(6):4166-70. doi: 10.1074/jbc.275.6.4166. J Biol Chem. 2000. PMID: 10660578
-
Measuring the modulatory effects of RGS proteins on GIRK channels.Methods Enzymol. 2004;389:131-54. doi: 10.1016/S0076-6879(04)89009-8. Methods Enzymol. 2004. PMID: 15313564 Review.
-
The Roles of Gβγ and Gα in Gating and Regulation of GIRK Channels.Int Rev Neurobiol. 2015;123:27-85. doi: 10.1016/bs.irn.2015.06.001. Epub 2015 Jul 26. Int Rev Neurobiol. 2015. PMID: 26422982 Review.
Cited by
-
Mouse models of GNAO1-associated movement disorder: Allele- and sex-specific differences in phenotypes.PLoS One. 2019 Jan 25;14(1):e0211066. doi: 10.1371/journal.pone.0211066. eCollection 2019. PLoS One. 2019. Retraction in: PLoS One. 2021 Oct 14;16(10):e0258912. doi: 10.1371/journal.pone.0258912. PMID: 30682176 Free PMC article. Retracted.
-
Desensitization of functional µ-opioid receptors increases agonist off-rate.Mol Pharmacol. 2014 Jul;86(1):52-61. doi: 10.1124/mol.114.092098. Epub 2014 Apr 18. Mol Pharmacol. 2014. PMID: 24748657 Free PMC article.
-
A role for RGS10 in beta-adrenergic modulation of G-protein-activated K+ (GIRK) channel current in rat atrial myocytes.J Physiol. 2008 Apr 15;586(8):2049-60. doi: 10.1113/jphysiol.2007.148346. Epub 2008 Feb 14. J Physiol. 2008. PMID: 18276732 Free PMC article.
-
A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade.J Neurosci. 2004 Jun 23;24(25):5684-93. doi: 10.1523/JNEUROSCI.0492-04.2004. J Neurosci. 2004. PMID: 15215290 Free PMC article.
-
Naringin directly activates inwardly rectifying potassium channels at an overlapping binding site to tertiapin-Q.Br J Pharmacol. 2011 Jul;163(5):1017-33. doi: 10.1111/j.1476-5381.2011.01315.x. Br J Pharmacol. 2011. PMID: 21391982 Free PMC article.
References
-
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–452. - PubMed
-
- Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ, Siderovski DP. Gβ association and effector interaction selectivities of the divergent Gγ subunit Gγ13. Journal of Biological Chemistry. 2001;276:49267–49274. - PubMed
-
- Burgon PG, Lee WL, Nixon AB, Peralta EG, Casey PJ. Phosphorylation and nuclear translocation of a regulator of G protein signaling (RGS10) Journal of Biological Chemistry. 2001;276:32828–32834. - PubMed
-
- Chase DL, Patikoglou GA, Koelle MR. Two RGS proteins that inhibit Gαo and Gαq signaling in C. elegans neurons require a Gβ5-like subunit for function. Current Biology. 2001;11:222–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

