Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels
- PMID: 12456820
- PMCID: PMC2290681
- DOI: 10.1113/jphysiol.2002.021881
Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels
Erratum in
- J Physiol. 2003 Feb 1;546(Pt 3):955
Retraction in
-
Retraction: Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels.J Physiol. 2015 May 1;593(9):2247. doi: 10.1113/JP270442. J Physiol. 2015. PMID: 26120649 Free PMC article. No abstract available.
Abstract
A calmodulin (CaM) binding 'IQ' domain on the L-type Ca(2+) channel (LTCC) C terminus and calmodulin kinase II (CaMK) both signal increases in LTCC opening probability (P(o)) by shifting LTCCs into a gating mode (mode 2) with long openings through a process called facilitation. However, the mechanism whereby CaMK and the IQ domain are targeted to LTCCs is unknown. Endogenous CaMK is targeted to LTCCs in excised cell membrane patches because LTCC P(o) increased significantly in CaM-enriched (20 microM) bath solution and this effect was prevented by a specific CaMK inhibitory peptide, but not by an inactive control peptide. Pre-exposure of myocytes to the cytoskeletal disrupting agents nocodazole (microtubule specific) or cytochalasin D (microfilament specific) prevented the effects of CaM-dependent increases in P(o) of LTCCs in excised membrane patches. Neither cytochalasin D nor nocodazole altered the distribution of LTCC gating modes under basal conditions in on-cell mode or excised cell membrane patches, but each of these agents occluded the response of LTCCs to exogenous, constitutively active CaMK and to an IQ-mimetic peptide (IQmp). Cytochalasin D and nocodazole pretreatment also prevented LTCC facilitation that followed a cell membrane depolarizing prepulse. In contrast, cytochalasin D and nocodazole did not affect the increase in LTCC P(o) or prevent the shift to mode 2 gating in response to protein kinase A, indicating that cytoskeletal disruption specifically prevents prepulse, CaMK and IQ-dependent LTCC facilitation.
Figures


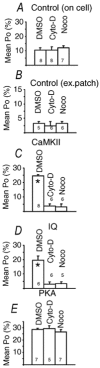

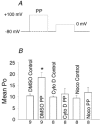
Comment in
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2014 Dec 5:NOT-OD-15-031. NIH Guide Grants Contracts (Bethesda). 2014. PMID: 25528784 Free PMC article. No abstract available.
References
-
- Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circulation Research. 1994;75:854–861. - PubMed
-
- Armstrong DL, Rossier MF, Shcherbatko AD, White RE. Enzymatic gating of voltage-activated calcium channels. Annals of the New York Academy of Sciences. 1991;635:26–34. - PubMed
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods in Cell Biology. 1994;40:3–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

