A role for the redox site in the modulation of the NMDA receptor by light
- PMID: 12456823
- PMCID: PMC2290695
- DOI: 10.1113/jphysiol.2002.032755
A role for the redox site in the modulation of the NMDA receptor by light
Abstract
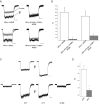
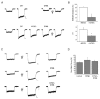
Light has been shown to modulate NMDA receptor function. In this study, we have performed experiments aimed at elucidating the putative site of action of light within the receptor structure. Whole-cell recordings were performed in Chinese hamster ovary cells expressing various combinations of NMDA receptor subunits. Although there was no apparent difference in the actions of light between wild-type NR1-NR2A and NR1-NR2B subunit configurations, the light enhancement of NMDA-induced currents was either completely abolished or substantially diminished in the redox site mutants NR1a (C744A, C798A)-NR2B and NR1a (C744A, C798A)-NR2A. Further studies demonstrated that chemical reduction of NR1a-NR2B NMDA receptors decreased its sensitivity to light. In addition, sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES), used to irreversibly bind free sulfhydryl groups and inactivate the redox site, abolished the effects of light on wild-type receptors. In contrast, no free sulfhydryls were available for MTSES following light stimulation, suggesting that light itself could not reduce the redox modulatory site. Our results suggest that a functionally intact, oxidized redox site is necessary for light-induced potentiation. Hence, light and redox modulation of the NMDA receptor may share a common intramolecular pathway for altering the function of this ion channel.
Figures
References
-
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. - PubMed
-
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. - PubMed
-
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-d-aspartate receptor subunits. Journal of Pharmacology and Experimental Therapeutics. 1996;279:515–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

