Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation
- PMID: 12456824
- PMCID: PMC2290684
- DOI: 10.1113/jphysiol.2002.029561
Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation
Abstract
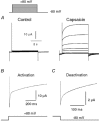
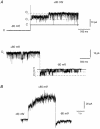
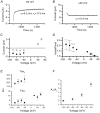
The responses of vanilloid receptor (VR) channels to changing membrane potential were studied in Xenopus oocytes and rat dorsal root ganglion (DRG) neurons. In oocytes, capsaicin-evoked VR currents increased instantaneously upon a step depolarization and thereafter rose biexponentially with time constants of approximately 20 and 1000 ms. Similarly, upon repolarization the current abruptly decreased, followed by a biexponential decay with time constants of approximately 4 and 200 ms. Qualitatively similar effects were observed in single channel recordings of native VR channels from DRG neurons and with endogenous VR activators, including heat (43 degrees C), H(+), anandamide and protein kinase C (PKC). The magnitude of the time-dependent current rise increased with membrane depolarization. This effect was accompanied by an increase in the relative proportion of the fast kinetic component, A(1). In contrast, the time constants of the activation and deactivation processes were not strongly voltage dependent. Increasing the agonist concentration both reduced the magnitude of the current rise and increased its overall rate, without significantly altering the deactivation rate. In contrast, PKC both speeded the current rise and slowed its decay. These results suggest that voltage interacts with agonists in a synergistic manner to augment VR current and this mechanism will be enhanced under conditions of inflammation when VRs are likely to be phosphorylated.
Figures




References
-
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. Journal of Neurophysiology. 2001;85:630–643. - PubMed
-
- Ahern GP, Kessler M, Premkumar LS. Voltage-dependence of the capsaicin receptor channel (VR1) Society for Neuroscience Abstracts. 2000;26:39.
-
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annual Review of Neuroscience. 2001;24:487–517. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

