The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex
- PMID: 12456831
- PMCID: PMC2290677
- DOI: 10.1113/jphysiol.2002.022103
The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex
Abstract
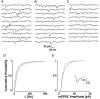
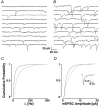
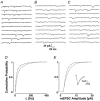
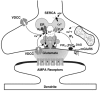
Loading slices of rat barrel cortex with 50 microM BAPTA-AM while recording from pyramidal cells in layer II induces a marked reduction in both the frequency and amplitudes of mEPSCs. These changes are due to a presynaptic action. Blocking the refilling of Ca(2+) stores with 20 microM cyclopiazonic acid (CPA), a SERCA pump inhibitor, in conjunction with neuronal depolarisation to activate Ca(2+) stores, results in a similar reduction of mEPSCs to that observed with BAPTA-AM, indicating that the source for intracellular Ca(2+) is the endoplasmic reticulum. Block or activation of ryanodine receptors by 20 microM ryanodine or 10 mM caffeine, respectively, shows that a significant proportion of mEPSCs are caused by Ca(2+) release from ryanodine stores. Blocking IP(3) receptors with 14 microM 2-aminoethoxydiphenylborane (2APB) also reduces the frequency and amplitude of mEPSCs, indicating the involvement of IP(3) stores in the generation of mEPSCs. Activation of group I metabotropic receptors with 20 microM (RS)-3,5-dihydroxyphenylglycine (DHPG) results in a significant increase in the frequency of mEPSCs, further supporting the role of IP(3) receptors and indicating a role of group I metabotropic receptors in causing transmitter release. Statistical evidence is presented for Ca(2+)-induced Ca(2+) release (CICR) from ryanodine stores after the spontaneous opening of IP(3) stores.
Figures





Similar articles
-
Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release.J Neurosci. 2009 May 20;29(20):6568-79. doi: 10.1523/JNEUROSCI.0181-09.2009. J Neurosci. 2009. PMID: 19458227 Free PMC article.
-
Investigation of the role of intracellular Ca(2+) stores in generation of the muscarinic agonist-induced slow afterdepolarization (sADP) in guinea-pig olfactory cortical neurones in vitro.Br J Pharmacol. 2000 Apr;129(7):1447-57. doi: 10.1038/sj.bjp.0703236. Br J Pharmacol. 2000. PMID: 10742301 Free PMC article.
-
Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum.Am J Physiol Lung Cell Mol Physiol. 2012 Jul;303(2):L161-8. doi: 10.1152/ajplung.00348.2011. Epub 2012 May 11. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22582116 Free PMC article.
-
Presence and functional significance of presynaptic ryanodine receptors.Prog Neurobiol. 2003 Apr;69(6):391-418. doi: 10.1016/s0301-0082(03)00053-4. Prog Neurobiol. 2003. PMID: 12880633 Review.
-
Neuronal calcium signaling.Neuron. 1998 Jul;21(1):13-26. doi: 10.1016/s0896-6273(00)80510-3. Neuron. 1998. PMID: 9697848 Review. No abstract available.
Cited by
-
Mitochondrial Dysfunction and Synaptic Transmission Failure in Alzheimer's Disease.J Alzheimers Dis. 2017;57(4):1071-1086. doi: 10.3233/JAD-160702. J Alzheimers Dis. 2017. PMID: 27662318 Free PMC article. Review.
-
Noradrenaline Increases mEPSC Frequency in Pyramidal Cells in Layer II of Rat Barrel Cortex via Calcium Release From Presynaptic Stores.Front Cell Neurosci. 2018 Jul 27;12:213. doi: 10.3389/fncel.2018.00213. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30100867 Free PMC article.
-
Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells.J Neurosci. 2006 Jan 4;26(1):86-95. doi: 10.1523/JNEUROSCI.2258-05.2006. J Neurosci. 2006. PMID: 16399675 Free PMC article.
-
Maintenance of homeostatic plasticity at the Drosophila neuromuscular synapse requires continuous IP3-directed signaling.Elife. 2019 Jun 10;8:e39643. doi: 10.7554/eLife.39643. Elife. 2019. PMID: 31180325 Free PMC article.
-
Ca2+-dependent mechanisms of presynaptic control at central synapses.Neuroscientist. 2006 Aug;12(4):317-26. doi: 10.1177/1073858405284672. Neuroscientist. 2006. PMID: 16840708 Free PMC article. Review.
References
-
- Bird MM. Presynaptic and postsynaptic organelles of synapses formed in cultures of previously dissociated mouse spinal cord. Cell and Tissue Research. 1978;194:503–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

