A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction
- PMID: 12456837
- PMCID: PMC2290674
- DOI: 10.1113/jphysiol.2002.024331
A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction
Abstract
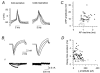
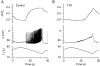
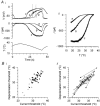
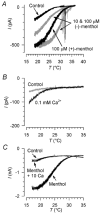
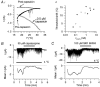
Skin temperature is sensed by peripheral thermoreceptors. Using the neuronal soma in primary culture as a model of the receptor terminal, we have investigated the mechanisms of cold transduction in thermoreceptive neurones from rat dorsal root ganglia. Cold-sensitive neurones were pre-selected by screening for an increase in [Ca(2+)](i) on cooling; 49 % of them were also excited by 0.5 microM capsaicin. Action potentials and voltage-gated currents of cold-sensitive neurones were clearly distinct from those of cold-insensitive neurones. All cold-sensitive neurones expressed an inward current activated by cold and sensitised by (-)-menthol, which was absent from cold-insensitive neurones. This current was carried mainly by Na(+) ions and caused a depolarisation on cooling accompanied by action potentials, inducing voltage-gated Ca(2+) entry; a minor fraction of Ca(2+) entry was voltage-independent. Application of (-)-menthol shifted the threshold temperatures of the cold-induced depolarisation and the inward current to the same extent, indicating that the cold- and menthol-activated current normally sets the threshold temperature for depolarisation during cooling. The action of menthol was stereospecific, with the (+)-isomer being a less effective agonist than the (-)-isomer. Extracellular Ca(2+) modulated the cold- and menthol-activated current in a similar way to its action on intact cold receptors: lowered [Ca(2+)](o) sensitised the current, while raised [Ca(2+)](o) antagonised the menthol-induced sensitisation. During long cooling pulses the current showed adaptation, which depended on extracellular Ca(2+) and was mediated by a rise in [Ca(2+)](i). This adaptation consisted of a shift in the temperature sensitivity of the channel. In capsaicin-sensitive neurones, capsaicin application caused a profound depression of the cold-activated current. Inclusion of nerve growth factor in the culture medium shifted the threshold of the cold-activated current towards warmer temperatures. The current was blocked by 50 microM capsazepine and 100 microM SKF 96365. We conclude that the cold- and menthol-activated current is the major mechanism responsible for cold-induced depolarisation in DRG neurones, and largely accounts for the known transduction properties of intact cold receptors.
Figures

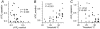




References
-
- Babes A, Amuzescu B, Krause U, Scholz A, Flonta M-L, Reid G. Cooling inhibits capsaicin-induced currents in cultured rat dorsal root ganglion neurones. Neuroscience Letters. 2002;317:131–134. - PubMed
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. Journal of Neurophysiology. 1969;32:1025–1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

