Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses
- PMID: 12456878
- PMCID: PMC138536
- DOI: 10.1073/pnas.252637799
Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses
Abstract
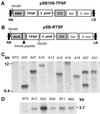
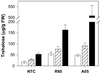
Trehalose is a nonreducing disaccharide of glucose that functions as a compatible solute in the stabilization of biological structures under abiotic stress in bacteria, fungi, and invertebrates. With the notable exception of the desiccation-tolerant "resurrection plants," trehalose is not thought to accumulate to detectable levels in most plants. We report here the regulated overexpression of Escherichia coli trehalose biosynthetic genes (otsA and otsB) as a fusion gene for manipulating abiotic stress tolerance in rice. The fusion gene has the advantages of necessitating only a single transformation event and a higher net catalytic efficiency for trehalose formation. The expression of the transgene was under the control of either tissue-specific or stress-dependent promoters. Compared with nontransgenic rice, several independent transgenic lines exhibited sustained plant growth, less photo-oxidative damage, and more favorable mineral balance under salt, drought, and low-temperature stress conditions. Depending on growth conditions, the transgenic rice plants accumulate trehalose at levels 3-10 times that of the nontransgenic controls. The observation that peak trehalose levels remain well below 1 mgg fresh weight indicates that the primary effect of trehalose is not as a compatible solute. Rather, increased trehalose accumulation correlates with higher soluble carbohydrate levels and an elevated capacity for photosynthesis under both stress and nonstress conditions, consistent with a suggested role in modulating sugar sensing and carbohydrate metabolism. These findings demonstrate the feasibility of engineering rice for increased tolerance of abiotic stress and enhanced productivity through tissue-specific or stress-dependent overproduction of trehalose.
Figures



Similar articles
-
Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth.Plant Physiol. 2003 Feb;131(2):516-24. doi: 10.1104/pp.007237. Plant Physiol. 2003. PMID: 12586876 Free PMC article.
-
Trehalose biosynthesis in response to abiotic stresses.J Integr Plant Biol. 2008 Oct;50(10):1223-9. doi: 10.1111/j.1744-7909.2008.00736.x. J Integr Plant Biol. 2008. PMID: 19017109 Review.
-
Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice.Planta. 2011 Nov;234(5):1007-18. doi: 10.1007/s00425-011-1458-0. Epub 2011 Jun 24. Planta. 2011. PMID: 21698458
-
A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis.Planta. 2007 Nov;226(6):1411-21. doi: 10.1007/s00425-007-0579-y. Epub 2007 Jul 13. Planta. 2007. PMID: 17628825
-
New insights on trehalose: a multifunctional molecule.Glycobiology. 2003 Apr;13(4):17R-27R. doi: 10.1093/glycob/cwg047. Epub 2003 Jan 22. Glycobiology. 2003. PMID: 12626396 Review.
Cited by
-
Identification of conserved drought-adaptive genes using a cross-species meta-analysis approach.BMC Plant Biol. 2015 May 3;15:111. doi: 10.1186/s12870-015-0493-6. BMC Plant Biol. 2015. PMID: 25935420 Free PMC article.
-
The Nicotiana tabacum L. major latex protein-like protein 423 (NtMLP423) positively regulates drought tolerance by ABA-dependent pathway.BMC Plant Biol. 2020 Oct 16;20(1):475. doi: 10.1186/s12870-020-02690-z. BMC Plant Biol. 2020. PMID: 33066728 Free PMC article.
-
A comprehensive analysis of Trehalose-6-phosphate synthase (TPS) gene for salinity tolerance in chickpea (Cicer arietinum L.).Sci Rep. 2022 Sep 29;12(1):16315. doi: 10.1038/s41598-022-20771-x. Sci Rep. 2022. PMID: 36175531 Free PMC article.
-
Bioinformatic analysis of epigenetic and microRNA mediated regulation of drought responsive genes in rice.PLoS One. 2012;7(11):e49331. doi: 10.1371/journal.pone.0049331. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145152 Free PMC article.
-
An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars.Plant Methods. 2011 Dec 30;7(1):49. doi: 10.1186/1746-4811-7-49. Plant Methods. 2011. PMID: 22206592 Free PMC article.
References
-
- Boyer J. S. (1982) Science 218, 443-448. - PubMed
-
- Hare P. D., Cress, W. A. & van Staden, J. (1998) Plant Cell Environ. 21, 535-553.
-
- Yancey P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. (1982) Science 217, 1214-1222. - PubMed
-
- Crowe J. H., Hoekstra, F. A. & Crowe, L. M. (1992) Annu. Rev. Physiol. 54, 579-599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

