A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata
- PMID: 12456884
- PMCID: PMC138613
- DOI: 10.1073/pnas.252427999
A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata
Abstract
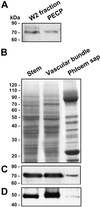
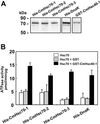
Plasmodesmata establish a pathway for the trafficking of non-cell-autonomously acting proteins and ribonucleoprotein complexes. Plasmodesmal enriched cell fractions and the contents of enucleate sieve elements, in the form of phloem sap, were used to isolate and characterize heat shock cognate 70 (Hsc70) chaperones associated with this cell-to-cell transport pathway. Three Cucurbita maxima Hsc70 chaperones were cloned and functional and sequence analysis led to the identification of a previously uncharacterized subclass of non-cell-autonomous chaperones. The highly conserved nature of the heat shock protein 70 (Hsp70) family, in conjunction with mutant analysis, permitted the characterization of a motif that allows these Hsc70 chaperones to engage the plasmodesmal non-cell-autonomous translocation machinery. Proof of concept that this motif is necessary for Hsp70 gain-of-movement function was obtained through the engineering of a human Hsp70 that acquired the capacity to traffic through plasmodesmata. These results are discussed in terms of the roles likely played by this subclass of Hsc70 chaperones in the trafficking of non-cell-autonomous proteins.
Figures


References
-
- Hartl F. U. & Hayer-Hartl, M. (2002) Science 295, 1852-1858. - PubMed
-
- Parsell D. A. & Lindquist, S. (1993) Annu. Rev. Genet. 27, 437-496. - PubMed
-
- Rutherford S. L. & Lindquist, S. (1998) Nature 396, 336-342. - PubMed
-
- Queitsch C., Sangster, T. A. & Lindquist, S. (2002) Nature 417, 618-624. - PubMed
-
- Pratt W. B., Krishna, P. & Olsen, L. J. (2001) Trends Plant Sci. 6, 54-58. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

