Carbohydrate-based particles: a new adjuvant for allergen-specific immunotherapy
- PMID: 12460198
- PMCID: PMC1782826
- DOI: 10.1046/j.1365-2567.2002.01535.x
Carbohydrate-based particles: a new adjuvant for allergen-specific immunotherapy
Abstract
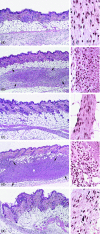
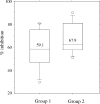
The occurrence of systemic anaphylactic side-effects in the course of allergen-specific immunotherapy has been strongly reduced by the adsorption of allergens to aluminium hydroxide, the most frequently used adjuvant in humans. Using the major timothy grass pollen allergen, Phl p 5b, in its recombinant form for immunization of mice, we demonstrate that carbohydrate-based particles (CBP) exhibit several potential advantages over aluminium-hydroxide as adjuvant for immunotherapy. Similar to alum-bound rPhl p 5b, CBP-bound rPhl p 5b induced a stronger antibody and cytokine response than unbound rPhl p 5b after subcutaneous injection in mice. The antibodies induced by CBP-bound rPhl p 5b, exhibited potentially beneficial activities as they cross-reacted with group 5 allergens from five other grass species and inhibited the binding of grass pollen allergic patients IgE to Phl p 5b. Alum-bound rPhl p 5b induced a preferential allergen-specific Th2-response characterized by high immunoglobulin G1 (IgG1) antibody levels and elevated interleukin (IL)-4 and IL-5 production in cultured splenocytes. By contrast, CBP-bound rPhl p 5b, but not rPhl p 5b alone or coadministered with CBP, induced a mixed allergen-specific T helper 1 (Th1)/Th2 immune response characterized by the additional production of allergen-specific IgG2a/b antibody responses and elevated interferon-gamma production. Conjugation of rPhl p 5b to CBP yielded a stable vaccine formulation with preserved immunogenic features of the allergen and, in contrast to alum, induced no granulomatous tissue reactions. Based on these results, CBP is suggested as a potentially useful adjuvant for specific immunotherapy of IgE-mediated allergies.
Figures
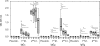


References
-
- Noon L. Prophylactic inoculation against hayfever. Lancet. 1911;1:1572.
-
- Sledge RF. Treatment of hay-fever with alum-precipitated pollen. US Naval Med Bull. 1938;36:18.
-
- Zoss AR, Koch CA, Hirose R. Alum-ragweed precipitate: preparation and clinical investigation; preliminary report. J Allergy Clin Immunol. 1939;8:29.
-
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. - PubMed
-
- Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

