Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase
- PMID: 12460985
- PMCID: PMC2173377
- DOI: 10.1083/jcb.200112131
Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase
Abstract
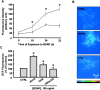
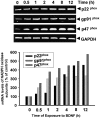
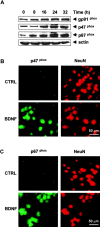
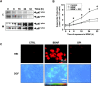
Several lines of evidence suggest that neurotrophins (NTs) potentiate or cause neuronal injury under various pathological conditions. Since NTs enhance survival and differentiation of cultured neurons in serum or defined media containing antioxidants, we set out experiments to delineate the patterns and underlying mechanisms of brain-derived neurotrophic factor (BDNF)-induced neuronal injury in mixed cortical cell cultures containing glia and neurons in serum-free media without antioxidants, where the three major routes of neuronal cell death, oxidative stress, excitotoxicity, and apoptosis, have been extensively studied. Rat cortical cell cultures, after prolonged exposure to NTs, underwent widespread neuronal necrosis. BDNF-induced neuronal necrosis was accompanied by reactive oxygen species (ROS) production and was dependent on the macromolecular synthesis. cDNA microarray analysis revealed that BDNF increased the expression of cytochrome b558, the plasma membrane-spanning subunit of NADPH oxidase. The expression and activation of NADPH oxidase were increased after exposure to BDNF. The selective inhibitors of NADPH oxidase prevented BDNF-induced ROS production and neuronal death without blocking antiapoptosis action of BDNF. The present study suggests that BDNF-induced expression and activation of NADPH oxidase cause oxidative neuronal necrosis and that the neurotrophic effects of NTs can be maximized under blockade of the pronecrotic action.
Figures


References
-
- Alderson, R.F., A.L. Alterman, Y.A. Barde, and R.M. Lindsay. 1990. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 5:297–306. - PubMed
-
- Atwal, J.K., B. Massie, F.D. Miller, and D.R. Kaplan. 2000. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 27:265–277. - PubMed
-
- Bannister, J.V., P. Bellavite, A. Davoli, P.J. Thornalley, and F. Rossi. 1982. The generation of hydroxyl radicals following superoxide production by neutrophil NADPH oxidase. FEBS Lett. 150:300–302. - PubMed
-
- Barde, Y.A. 1994. Neurotrophins: a family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 390:45–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

