Telomerase activation and rejuvenation of telomere length in stimulated T cells derived from serially transplanted hematopoietic stem cells
- PMID: 12461078
- PMCID: PMC2194261
- DOI: 10.1084/jem.20021003
Telomerase activation and rejuvenation of telomere length in stimulated T cells derived from serially transplanted hematopoietic stem cells
Abstract
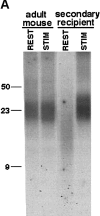
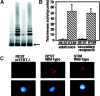
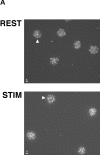
Telomeres shorten in hematopoietic cells, including hematopoietic stem cells (HSCs), during aging and after transplantation, despite the presence of readily detectable levels of telomerase in these cells. In T cells, antigenic stimulation has been shown to result in a marked increase in the level of telomerase activity. We now show that stimulation of T cells derived from serially transplanted HSC results in a telomerase-dependent elongation of telomere length to a size similar to that observed in T cells isolated directly from young mice. Southern analysis of telomere length in resting and anti-CD3/CD28 stimulated donor-derived splenic T cells revealed an increase in telomere size by approximately 7 kb for the population as a whole. Stimulation of donor-derived T cells from recipients of HSCs from telomerase-deficient mice did not result in regeneration of telomere length, demonstrating a dependence on telomerase. Furthermore, clonal anti-CD3/CD28 stimulation of donor-derived T cells followed by fluorescent in situ hybridization (FISH) analysis of telomeric signal intensity showed that telomeres had increased in size by approximately 50% for all clonal expansions. Together, these results imply that one role for telomerase in T cells may be to renew or extend replicative potential via the rejuvenation of telomere length.
Figures




References
-
- Blasco, M., H.W. Lee, M.P. Hande, E. Samper, P.M. Lansdorp, R.A. DePinho, and C.W. Greider. 1996. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 91:25–34. - PubMed

