Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells
- PMID: 12461079
- PMCID: PMC2194269
- DOI: 10.1084/jem.20020493
Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells
Abstract
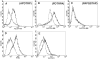
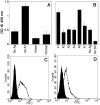
In contrast to protein antigens, processing of glycoproteins by dendritic cells (DCs) for presentation to T cells has not been well studied. We developed mouse T cell hybridomas to study processing and presentation of the tumor antigen MUC1 as a model glycoprotein. MUC1 is expressed on the surface as well as secreted by human adenocarcinomas. Circulating soluble MUC1 is available for uptake, processing, and presentation by DCs in vivo and better understanding of how that process functions in the case of glycosylated antigens may shed light on antitumor immune responses that could be initiated against this glycoprotein. We show that DCs endocytose MUC1 glycopeptides, transport them to acidic compartments, process them into smaller peptides, and present them on major histocompatability complex (MHC) class II molecules without removing the carbohydrates. Glycopeptides that are presented on DCs are recognized by T cells. This suggests that a much broader repertoire of T cells could be elicited against MUC1 and other glycoproteins than expected based only on their peptide sequences.
Figures


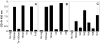
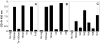




Similar articles
-
Presentation of MUC1 tumor antigen by class I MHC and CTL function correlate with the glycosylation state of the protein taken Up by dendritic cells.Cell Immunol. 1999 Jun 15;194(2):143-9. doi: 10.1006/cimm.1999.1512. Cell Immunol. 1999. PMID: 10383817
-
Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice.Biol Chem. 2009 Jul;390(7):611-8. doi: 10.1515/BC.2009.070. Biol Chem. 2009. PMID: 19426130 Free PMC article.
-
Antigen processing and presentation of a naturally glycosylated protein elicits major histocompatibility complex class II-restricted, carbohydrate-specific T cells.Eur J Immunol. 1996 Aug;26(8):1906-10. doi: 10.1002/eji.1830260835. Eur J Immunol. 1996. PMID: 8765038
-
Immunology of O-glycosylated proteins: approaches to the design of a MUC1 glycopeptide-based tumor vaccine.Curr Protein Pept Sci. 2006 Aug;7(4):307-15. doi: 10.2174/138920306778018034. Curr Protein Pept Sci. 2006. PMID: 16918445 Review.
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
Cited by
-
A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15029-34. doi: 10.1073/pnas.2432220100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657390 Free PMC article.
-
Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution.Blood. 2005 Jun 15;105(12):4583-9. doi: 10.1182/blood-2004-10-3848. Epub 2005 Mar 3. Blood. 2005. PMID: 15746083 Free PMC article.
-
Going Native: Synthesis of Glycoproteins and Glycopeptides via Native Linkages To Study Glycan-Specific Roles in the Immune System.Bioconjug Chem. 2019 Nov 20;30(11):2715-2726. doi: 10.1021/acs.bioconjchem.9b00588. Epub 2019 Oct 18. Bioconjug Chem. 2019. PMID: 31580646 Free PMC article. Review.
-
Neoantigens: promising targets for cancer therapy.Signal Transduct Target Ther. 2023 Jan 6;8(1):9. doi: 10.1038/s41392-022-01270-x. Signal Transduct Target Ther. 2023. PMID: 36604431 Free PMC article. Review.
-
An anti-MUC1-antibody-interleukin-2 fusion protein that activates resting NK cells to lysis of MUC1-positive tumour cells.Br J Cancer. 2003 Sep 15;89(6):1130-9. doi: 10.1038/sj.bjc.6601267. Br J Cancer. 2003. PMID: 12966437 Free PMC article.
References
-
- Lehner, P.J., and P. Cresswell. 1996. Processing and delivery of peptides presented by MHC class I molecules. Curr. Opin. Immunol. 8:59–67. - PubMed
-
- Germain, R.N., F. Castellino, R. Han, C. Reis e Sousa, P. Romagnoli, S. Sadegh-Nasseri, and G.M. Zhong. 1996. Processing and presentation of endocytically acquired protein antigens by MHC class II and class I molecules. Immunol. Rev. 151:5–30. - PubMed
-
- Pieters, J. 2000. MHC class II-restricted antigen processing and presentation. Adv. Immunol. 75:159–208. - PubMed
-
- Watts, C. 2001. Antigen processing in the endocytic compartment. Curr. Opin. Immunol. 13:26–31. - PubMed
-
- Harding, C.V. 1996. Class II antigen processing: analysis of compartments and functions. Crit. Rev. Immunol. 16:13–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

