GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression
- PMID: 12461080
- PMCID: PMC2194266
- DOI: 10.1084/jem.20020436
GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression
Abstract
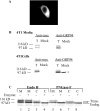
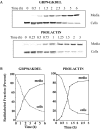
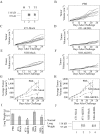
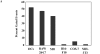
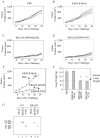
In chemical carcinogenesis models, GRP94 (gp96) elicits tumor-specific protective immunity. The tumor specificity of this response is thought to reflect immune responses to GRP94-bound peptide antigens, the cohort of which uniquely identifies the GRP94 tissue of origin. In this study, we examined the apparent tissue restriction of GRP94-elicited protective immunity in a 4T1 mammary carcinoma model. We report that the vaccination of BALB/c mice with irradiated fibroblasts expressing a secretory form of GRP94 markedly suppressed 4T1 tumor growth and metastasis. In addition, vaccination with irradiated cells secreting the GRP94 NH(2)-terminal geldanamycin-binding domain (NTD), a region lacking canonical peptide-binding motifs, yielded a similar suppression of tumor growth and metastatic progression. Conditioned media from cultures of GRP94 or GRP94 NTD-secreting fibroblasts elicited the up-regulation of major histocompatibility complex class II and CD86 in dendritic cell cultures, consistent with a natural adjuvant function for GRP94 and the GRP94 NTD. Based on these findings, we propose that GRP94-elicited tumor suppression can occur independent of the GRP94 tissue of origin and suggest a primary role for GRP4 natural adjuvant function in antitumor immune responses.
Figures




Similar articles
-
Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo.J Immunol. 2004 Apr 1;172(7):4195-203. doi: 10.4049/jimmunol.172.7.4195. J Immunol. 2004. PMID: 15034032
-
Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation.J Immunol. 2010 Dec 1;185(11):6819-30. doi: 10.4049/jimmunol.1000448. Epub 2010 Nov 3. J Immunol. 2010. PMID: 21048103 Free PMC article.
-
Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen.J Immunol. 2003 Mar 15;170(6):2912-22. doi: 10.4049/jimmunol.170.6.2912. J Immunol. 2003. PMID: 12626542
-
The messenger and the message: gp96 (GRP94)-peptide interactions in cellular immunity.Cell Stress Chaperones. 2004 Winter;9(4):325-31. doi: 10.1379/csc-62.1. Cell Stress Chaperones. 2004. PMID: 15633290 Free PMC article. Review.
-
The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells.Cell Stress Chaperones. 2000 Nov;5(5):462-70. doi: 10.1379/1466-1268(2000)005<0462:thspga>2.0.co;2. Cell Stress Chaperones. 2000. PMID: 11189453 Free PMC article. Review.
Cited by
-
Chaperone proteins and brain tumors: potential targets and possible therapeutics.Neuro Oncol. 2005 Jul;7(3):260-78. doi: 10.1215/S1152851704001188. Neuro Oncol. 2005. PMID: 16053701 Free PMC article. Review.
-
Hexose-6-phosphate dehydrogenase controls cancer cell proliferation and migration through pleiotropic effects on the unfolded-protein response, calcium homeostasis, and redox balance.FASEB J. 2018 May;32(5):2690-2705. doi: 10.1096/fj.201700870RR. Epub 2018 Jan 2. FASEB J. 2018. PMID: 29295867 Free PMC article.
-
Redundancy renders the glycoprotein 96 receptor scavenger receptor A dispensable for cross priming in vivo.Immunology. 2008 Dec;125(4):480-91. doi: 10.1111/j.1365-2567.2008.02861.x. Epub 2008 May 15. Immunology. 2008. PMID: 18489571 Free PMC article.
-
Malaria DNA vaccine gp96NTD-CSP elicits both CSP-specific antibody and CD8(+) T cell response.Parasitol Res. 2015 Jun;114(6):2333-9. doi: 10.1007/s00436-015-4429-8. Epub 2015 Mar 20. Parasitol Res. 2015. PMID: 25786609
-
Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice.World J Gastroenterol. 2005 May 21;11(19):2858-63. doi: 10.3748/wjg.v11.i19.2858. World J Gastroenterol. 2005. PMID: 15902719 Free PMC article.
References
-
- Srivastava, P.K. 2002. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 20:395–425. - PubMed
-
- Blachere, N.E., H. Udono, S. Janetzki, Z. Li, and P.K. Srivastava. 1993. Heat shock protein vaccines against cancer. J. Immunother. 14:352–356. - PubMed
-
- Tamura, Y., P. Peng, K. Liu, M. Daou, and P.K. Srivastava. 1997. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 278:117–120. - PubMed
-
- Srivastava, P.K., and M. Heike. 1991. Stress-induced proteins in immune response to cancer. Curr. Top. Microbiol. Immunol. 167:109–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

