Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation
- PMID: 12461086
- PMCID: PMC2194260
- DOI: 10.1084/jem.20011794
Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation
Abstract
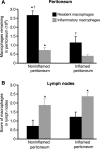
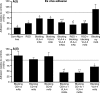
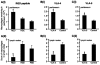
Macrophage clearance is essential for the resolution of inflammation. Much is known about how monocytes enter the inflammatory site but little is known about how resultant macro-phages are cleared. We have previously demonstrated that macrophage clearance from resolving peritonitis occurs by emigration into draining lymphatics rather than local apoptosis. We now examine mechanisms for this process, in particular by evaluating the hypothesis that modulation of adhesion interactions between macrophages and cells lining the lymphatics regulates the rate of macrophage clearance. We demonstrate in vivo that macrophages adhere specifically to mesothelium overlying draining lymphatics and that their emigration rate is regulated by the state of macrophage activation. We observed that macrophage-mesothelial adhesion is Arg-Gly-Asp (RGD) sensitive and partially mediated by very late antigen (VLA)-4 and VLA-5 but not alpha(v) or beta(2) integrins. Moreover, macrophage clearance into lymphatics can be blocked in vivo by RGD peptides and VLA-4 and VLA-5 but not beta(2) blocking antibodies. This is the first evidence that macrophage emigration from the inflamed site is controlled and demonstrates that this is exerted through specific adhesion molecule regulation of macrophage-mesothelial interactions. It highlights the importance of adhesion molecules governing entry of cells into the lymphatic circulation, thus opening a new avenue for manipulating the resolution of inflammation.
Figures


References
-
- Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5–S11. - PubMed
-
- Bellingan, G.J., H. Caldwell, S.E. Howie, I. Dransfield, and C. Haslett. 1996. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 157:2577–2585. - PubMed
-
- Van Furth, R. 1992. Development and distribution of mononuclear phagocytes. Inflammation: Basic Principles and Clinical Correlates. Second Edition. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 325–341.
-
- Adams, D.O., and T.A. Hamilton. 1992. Macrophages as destructive cells in host defence. Inflammation: Basic Principles and Clinical Correlates. Second Edition. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 637–643.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

