In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants
- PMID: 12461177
- PMCID: PMC138611
- DOI: 10.1073/pnas.252500999
In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants
Abstract
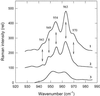
Dissipation of excess light energy in plant photosynthetic membranes plays an important role in the response of plants to the environment, providing short-term balancing between the intensity of sunlight and photosynthetic capacity. The carotenoid zeaxanthin and the photosystem II subunit PsbS play vital roles in this process, but the mechanism of their action is largely unexplained. Here we report that the isolated photosystem II subunit PsbS was able to bind exogenous zeaxanthin, the binding resulting in a strong red shift in the absorption spectrum, and the appearance of characteristic features in the resonance Raman spectrum and a distinct circular dichroism spectrum, indicating pigment-protein, as well as specific pigment-pigment, interaction. A strong shift in the absorption spectrum of PsbS phenylalanine residues after zeaxanthin binding was observed. It is concluded that zeaxanthin binding to PsbS is the origin of the well known energy dissipation-related 535-nm absorption change that we showed in vivo to arise from activation of 1-2 molecules of this pigment. The altered properties of zeaxanthin and PsbS that result from this interaction provide the first direct indication about how they regulate energy dissipation.
Figures
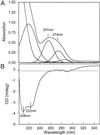
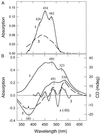
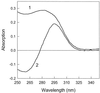
References
-
- Björkman O. & Demmig-Adams, B. (1995) in Ecophysiology of Photosynthesis: Ecological Studies, eds. Schulze, E. D. & Caldwell, M. M. (Springer, Berlin), pp. 14–47.
-
- Horton P., Murchie, E. H., Ruban, A. V. & Walters, R. G. (2001) Novartis Found. Symp. 236, 117-134. - PubMed
-
- Horton P., Ruban, A. V. & Walters, R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655-684. - PubMed
-
- Niyogi K. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333-360. - PubMed
-
- Külheim C., Ägren, J. & Jansson, S. (2002) Science 297, 91-93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

