Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines
- PMID: 12461787
- PMCID: PMC2572568
- DOI: 10.1002/jcb.10353
Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines
Abstract
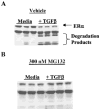
Normal mammary epithelial cells are rapidly induced to G(1) arrest by the widely expressed cytokine, transforming growth factor beta (TGF-beta1). Studies in established breast cancer cell lines that express the estrogen receptor alpha (ERalpha) have demonstrated loss of this responsiveness. This inverse correlation suggests interpathway signaling important to cell growth and regulation. The adenocarcinoma breast cell line BT474, which was not growth arrested by TGF-beta1, was used as a model of estrogen-inducible growth to explore interpathway crosstalk. Although BT474 cells were not growth-arrested by TGF-beta1 as determined by flow cytometry analysis and 5'-bromo-3'-deoxyuridine incorporation into DNA, estrogen receptor protein levels were attenuated by 100 pM TGF-beta1 after 6 h. This decrease in ERalpha reached 50% of untreated control levels by 24 h of treatment and was further supported by a 50% decrease in estrogen-inducible DNA synthesis. Inspection of ERalpha transcripts suggested that this decrease was primarily the result of altered ERalpha protein stability or availability. Use of the proteasome inhibitor, MG132, abolished all effects on ERalpha by TGF-beta1. Collectively, this data supports a role for TGF-beta1 in regulating the growth of otherwise insensitive breast cancer cells through modulation of ERalpha stability.
Copyright 2002 Wiley-Liss, Inc.
Figures
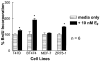
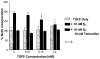

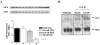
Similar articles
-
Hypoxia induces proteasome-dependent degradation of estrogen receptor alpha in ZR-75 breast cancer cells.Mol Endocrinol. 2002 Oct;16(10):2231-42. doi: 10.1210/me.2001-0347. Mol Endocrinol. 2002. PMID: 12351689
-
The NEDD8 pathway is required for proteasome-mediated degradation of human estrogen receptor (ER)-alpha and essential for the antiproliferative activity of ICI 182,780 in ERalpha-positive breast cancer cells.Mol Endocrinol. 2003 Mar;17(3):356-65. doi: 10.1210/me.2002-0323. Epub 2002 Dec 18. Mol Endocrinol. 2003. PMID: 12554766
-
Crosstalk between estrogen receptor alpha and the aryl hydrocarbon receptor in breast cancer cells involves unidirectional activation of proteasomes.FEBS Lett. 2000 Jul 28;478(1-2):109-12. doi: 10.1016/s0014-5793(00)01830-5. FEBS Lett. 2000. PMID: 10922479
-
On and off: proteasome and TGF-beta signaling.Exp Cell Res. 2003 Dec 10;291(2):275-81. doi: 10.1016/j.yexcr.2003.07.007. Exp Cell Res. 2003. PMID: 14644150 Review.
-
Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells.J Mammary Gland Biol Neoplasia. 2000 Jul;5(3):295-306. doi: 10.1023/a:1009550912337. J Mammary Gland Biol Neoplasia. 2000. PMID: 14973392 Review.
Cited by
-
Blockade of Autocrine TGF-β Signaling Inhibits Stem Cell Phenotype, Survival, and Metastasis of Murine Breast Cancer Cells.J Stem Cell Res Ther. 2012 Feb 19;2(1):1-8. doi: 10.4172/2157-7633.1000116. J Stem Cell Res Ther. 2012. PMID: 23482850 Free PMC article.
-
A Dynamic Shift in Estrogen Receptor Expression During Granulosa Cell Differentiation in the Ovary.Endocrinology. 2025 Jan 6;166(2):bqaf006. doi: 10.1210/endocr/bqaf006. Endocrinology. 2025. PMID: 39834231
-
Efficacy of hormonal suppression in a patient with chyluria due to lymphangioleiomyomatosis.Multidiscip Respir Med. 2011 Oct 31;6(5):313-7. doi: 10.1186/2049-6958-6-5-313. Multidiscip Respir Med. 2011. PMID: 22958860 Free PMC article.
-
Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells.Respir Res. 2018 Aug 30;19(1):160. doi: 10.1186/s12931-018-0861-5. Respir Res. 2018. PMID: 30165855 Free PMC article.
-
Alterations in macrophages and monocytes from tumor-bearing mice: evidence of local and systemic immune impairment.Immunol Res. 2013 Dec;57(1-3):86-98. doi: 10.1007/s12026-013-8438-3. Immunol Res. 2013. PMID: 24203436 Review.
References
-
-
American Cancer Society. 2001. Facts and figures.
-
-
- Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK. Transforming growth factor beta: Potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988;48:3898–3904. - PubMed
-
- Baudino TA, Kraichely DM, Jefcoat SC, Jr, Winchester SK, Partridge NC, MacDonald PN. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem. 1998;273:16434–16441. - PubMed
-
- Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;86:1403–1408. - PubMed
-
- Bonni S, Wang HR, Causing CG, Kavsak P, Stroschein SL, Luo K, Wrana JL. TGF-beta induces assembly of a Smad2–Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol. 2001;3:587–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

