Bile acid-induced Mallory body formation in drug-primed mouse liver
- PMID: 12466118
- PMCID: PMC1850910
- DOI: 10.1016/S0002-9440(10)64480-X
Bile acid-induced Mallory body formation in drug-primed mouse liver
Abstract
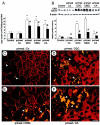
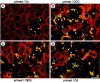
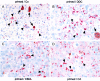
Chronic cholestasis is associated with retention of bile acids and profound cytoskeletal alterations in hepatocytes including Mallory body (MB) formation. The mechanisms responsible for MB formation in cholestatic liver diseases are unclear. The aim of our study was to determine the relevance of cholestasis and bile acids for MB formation. For this purpose mice received a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-supplemented diet for 2.5 months to induce MB formation. After recovery from DDC intoxication for 4 weeks followed by disappearance of MBs, these drug-primed mice were subjected to DDC refeeding, common bile duct ligation (CBDL), and feeding of a cholic acid (CA)-supplemented diet for 7 days, respectively. Cytokeratin (CK) 8 and CK 18 expression was studied by competitive reverse transcriptase-polymerase chain reaction and Western blot analysis. Cytoskeletal alterations of hepatocytes and MB formation were monitored by immunofluorescence microscopy and immunohistochemistry using CK-, ubiquitin-, and MB-specific antibodies. Like DDC refeeding, both CBDL and CA feeding of drug-primed mice significantly increased CK 8 and CK 18 mRNA and protein levels (with excess of CK 8) and resulted in ubiquitination and abnormal phosphorylation of CKs. Furthermore, CBDL and CA feeding resulted in rapid neoformation of MBs in drug-primed mice. It is concluded that MB formation in cholestatic liver diseases may be triggered by the action of potentially toxic bile acids.
Figures
Similar articles
-
Cytokeratins as targets for bile acid-induced toxicity.Am J Pathol. 2002 Feb;160(2):491-9. doi: 10.1016/S0002-9440(10)64868-7. Am J Pathol. 2002. PMID: 11839569 Free PMC article.
-
Mallory body formation in primary biliary cirrhosis is associated with increased amounts and abnormal phosphorylation and ubiquitination of cytokeratins.J Hepatol. 2003 Apr;38(4):387-94. doi: 10.1016/s0168-8278(02)00439-7. J Hepatol. 2003. PMID: 12663227
-
Sequence of events in the assembly of Mallory body components in mouse liver: clues to the pathogenesis and significance of Mallory body formation.J Hepatol. 2001 May;34(5):665-75. doi: 10.1016/s0168-8278(00)00099-4. J Hepatol. 2001. PMID: 11434612
-
Keratin 18 overexpression but not phosphorylation or filament organization blocks mouse Mallory body formation.Hepatology. 2007 Jan;45(1):88-96. doi: 10.1002/hep.21471. Hepatology. 2007. PMID: 17187412
-
Loss of hepatocyte Usp53 protects mice from a form of xenobiotic-induced liver injury.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167624. doi: 10.1016/j.bbadis.2024.167624. Epub 2024 Dec 19. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39705897 Review.
Cited by
-
Role of p62/SQSTM1 in liver physiology and pathogenesis.Exp Biol Med (Maywood). 2013 May;238(5):525-38. doi: 10.1177/1535370213489446. Exp Biol Med (Maywood). 2013. PMID: 23856904 Free PMC article. Review.
-
Expression and localization of sterile alpha motif domain containing 5 is associated with cell type and malignancy of biliary tree.PLoS One. 2017 Apr 7;12(4):e0175355. doi: 10.1371/journal.pone.0175355. eCollection 2017. PLoS One. 2017. PMID: 28388653 Free PMC article.
-
Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling.Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G91-G104. doi: 10.1152/ajpgi.00027.2015. Epub 2016 May 5. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27151938 Free PMC article.
-
Involvement of Autophagy in Ageing and Chronic Cholestatic Diseases.Cells. 2021 Oct 16;10(10):2772. doi: 10.3390/cells10102772. Cells. 2021. PMID: 34685751 Free PMC article. Review.
-
Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation.World J Gastroenterol. 2005 Jul 21;11(27):4167-72. doi: 10.3748/wjg.v11.i27.4167. World J Gastroenterol. 2005. PMID: 16015684 Free PMC article.
References
-
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R: The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31:11-24 - PubMed
-
- Denk H, Stumptner C, Zatloukal K: Mallory bodies revisited. J Hepatol 2000, 32:689-702 - PubMed
-
- Mallory FB: Cirrhosis of the liver: five different lesions from which it may arise. Bull Johns Hopkins Hosp 1911, 22:69-75
-
- Stumptner C, Fuchsbichler A, Lehner M, Zatloukal K, Denk H: Sequence of events in the assembly of Mallory body components in mouse liver: clues to the pathogenesis and significance of Mallory body formation. J Hepatol 2001, 34:665-675 - PubMed
-
- Zatloukal K, Böck G, Rainer I, Denk H, Weber K: High molecular weight components are main constituents of Mallory bodies isolated with fluorescence activated cell sorter. Lab Invest 1991, 64:200-206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

