Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis
- PMID: 12466131
- PMCID: PMC1850894
- DOI: 10.1016/S0002-9440(10)64493-8
Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis
Abstract
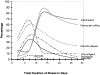
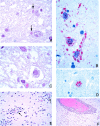
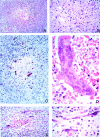
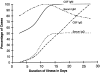
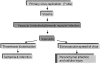
In 1998, an outbreak of acute encephalitis with high mortality rates among pig handlers in Malaysia led to the discovery of a novel paramyxovirus named Nipah virus. A multidisciplinary investigation that included epidemiology, microbiology, molecular biology, and pathology was pivotal in the discovery of this new human infection. Clinical and autopsy findings were derived from a series of 32 fatal human cases of Nipah virus infection. Diagnosis was established in all cases by a combination of immunohistochemistry (IHC) and serology. Routine histological stains, IHC, and electron microscopy were used to examine autopsy tissues. The main histopathological findings included a systemic vasculitis with extensive thrombosis and parenchymal necrosis, particularly in the central nervous system. Endothelial cell damage, necrosis, and syncytial giant cell formation were seen in affected vessels. Characteristic viral inclusions were seen by light and electron microscopy. IHC analysis showed widespread presence of Nipah virus antigens in endothelial and smooth muscle cells of blood vessels. Abundant viral antigens were also seen in various parenchymal cells, particularly in neurons. Infection of endothelial cells and neurons as well as vasculitis and thrombosis seem to be critical to the pathogenesis of this new human disease.
Figures






References
-
- : Centers for Disease Control and Prevention: Outbreak of Hendra-like virus-Malaysia and Singapore, 1998–1999. MMWR Morb Mortal Wkly Rep 1999, 48:265-269 - PubMed
-
- Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT: Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354:1257-1259 - PubMed
-
- Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, Chew SK, Ang B, Rollin PE, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek TG: Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 1999, 354:1253-1256 - PubMed
-
- Tambyah PA, Tan JH, Ong BK, Ho KH, Chan KP: First case of Nipah virus encephalitis in Singapore. Intern Med J 2001, 31:132-133 - PubMed
-
- Parashar UD, Sunn LM, Ong F, Mounts AW, Arif MT, Ksiazek TG, Kamaluddin MA, Mustafa AN, Kaur H, Ding LM, Othman G, Radzi HM, Kitsutani PT, Stockton PC, Arokiasamy J, Gary HE, Jr, Anderson LJ: Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J Infect Dis 2000, 181:1755-1759 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

