'Cyclic alopecia' in Msx2 mutants: defects in hair cycling and hair shaft differentiation
- PMID: 12466204
- PMCID: PMC4386654
- DOI: 10.1242/dev.00201
'Cyclic alopecia' in Msx2 mutants: defects in hair cycling and hair shaft differentiation
Abstract
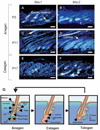
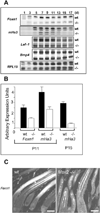
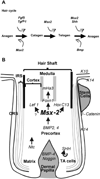
Msx2-deficient mice exhibit progressive hair loss, starting at P14 and followed by successive cycles of wavelike regrowth and loss. During the hair cycle, Msx2 deficiency shortens anagen phase, but prolongs catagen and telogen. Msx2-deficient hair shafts are structurally abnormal. Molecular analyses suggest a Bmp4/Bmp2/Msx2/Foxn1 acidic hair keratin pathway is involved. These structurally abnormal hairs are easily dislodged in catagen implying a precocious exogen. Deficiency in Msx2 helps to reveal the distinctive skin domains on the same mouse. Each domain cycles asynchronously - although hairs within each skin domain cycle in synchronized waves. Thus, the combinatorial defects in hair cycling and differentiation, together with concealed skin domains, account for the cyclic alopecia phenotype.
Figures




Similar articles
-
Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation.Dev Biol. 2009 Feb 15;326(2):420-30. doi: 10.1016/j.ydbio.2008.11.021. Epub 2008 Dec 7. Dev Biol. 2009. PMID: 19103190 Free PMC article.
-
Gsdma3 is required for hair follicle differentiation in mice.Biochem Biophys Res Commun. 2010 Dec 3;403(1):18-23. doi: 10.1016/j.bbrc.2010.10.094. Epub 2010 Oct 25. Biochem Biophys Res Commun. 2010. PMID: 20977888
-
Hairless down-regulates expression of Msx2 and its related target genes in hair follicles.J Dermatol Sci. 2013 Sep;71(3):203-9. doi: 10.1016/j.jdermsci.2013.04.019. Epub 2013 Apr 28. J Dermatol Sci. 2013. PMID: 23702391
-
Mouse models of alopecia: identifying structural genes that are baldly needed.Trends Mol Med. 2003 Feb;9(2):79-84. doi: 10.1016/s1471-4914(02)00025-4. Trends Mol Med. 2003. PMID: 12615042 Review.
-
Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity.Int J Dev Biol. 2009;53(5-6):857-68. doi: 10.1387/ijdb.072564mp. Int J Dev Biol. 2009. PMID: 19378257 Free PMC article. Review.
Cited by
-
Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes.Proc Natl Acad Sci U S A. 2006 Nov 28;103(48):18166-71. doi: 10.1073/pnas.0608899103. Epub 2006 Nov 17. Proc Natl Acad Sci U S A. 2006. PMID: 17114283 Free PMC article.
-
The critical roles of serum/glucocorticoid-regulated kinase 3 (SGK3) in the hair follicle morphogenesis and homeostasis: the allelic difference provides novel insights into hair follicle biology.Am J Pathol. 2006 Apr;168(4):1119-33. doi: 10.2353/ajpath.2006.050507. Am J Pathol. 2006. PMID: 16565488 Free PMC article.
-
Foxn1 in Skin Development, Homeostasis and Wound Healing.Int J Mol Sci. 2018 Jul 4;19(7):1956. doi: 10.3390/ijms19071956. Int J Mol Sci. 2018. PMID: 29973508 Free PMC article. Review.
-
Barx2 functions through distinct corepressor classes to regulate hair follicle remodeling.Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3708-13. doi: 10.1073/pnas.0500519102. Epub 2005 Feb 22. Proc Natl Acad Sci U S A. 2005. PMID: 15728386 Free PMC article.
-
Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules.Int J Mol Sci. 2019 Jul 13;20(14):3447. doi: 10.3390/ijms20143447. Int J Mol Sci. 2019. PMID: 31337050 Free PMC article.
References
-
- Baxter RM, Brissette JL. Role of the nude gene in epithelial terminal differentiation. J. Invest. Dermatol. 2002;118:303–309. - PubMed
-
- Bechtold LS. Ultra-structural evaluation of mouse mutations. In: Sundberg JP, Boggess D, editors. Systematic Approach to Evaluation of Mouse Mutations. Boca Raton, FL: CRC Press; 2000. pp. 121–129.
-
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1999;1:158–164. - PubMed
-
- Brissette JL, Li J, Kamimura J, Lee D, Dotto GP. The product of the mouse nude locus, Foxn1, regulates the balance between epithelial cell growth and differentiation. Genes Dev. 1996;10:2212–2221. - PubMed
-
- Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat. Genet. 1999;21:410–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

