Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels
- PMID: 12466226
- PMCID: PMC1573614
- DOI: 10.1038/sj.bjp.0704989
Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels
Abstract
1. KCNQ K(+) channels are thought to underlie the M current of neurons. To probe if the KCNQ2 and KCNQ3 subtypes underlie the M current of rat superior cervical ganglia (SCG) neurons and of hippocampus, we raised specific antibodies against them and also used the cysteine-alkylating agent N-ethylmaleimide (NEM) as an additional probe of subunit composition. 2. Tested on tsA-201 (tsA) cells transfected with cloned KCNQ1-5 subunits, our antibodies showed high affinity and selectivity for the appropriate subtype. The antibodies immunostained SCG neurons and hippocampal sections at levels similar to those for channels expressed in tsA cells, indicating that KCNQ2 and KCNQ3 are present in SCG and hippocampal neurons. Some hippocampal regions contained only KCNQ2 or KCNQ3 subunits, suggesting the presence of M currents produced by channels other than KCNQ2/3 heteromultimers. 3. We found that NEM augmented M currents in SCG neurons and KCNQ2/3 currents in tsA cells via strong voltage-independent and modest voltage-dependent actions. Expression of individual KCNQ subunits in tsA cells revealed voltage-independent augmentation of KCNQ2, but not KCNQ1 nor KCNQ3, currents by NEM indicating that this action on SCG M currents likely localizes to KCNQ2. Much of the voltage-independent action is lost after the C242T mutation in KCNQ2. 4. The correspondence of NEM effects on expressed KCNQ2/3 and SCG M currents, along with the antibody labelling, provide further evidence that KCNQ2 and KCNQ3 subunits strongly contribute to the M current of neurons. The site of NEM action may be important for treatment of diseases caused by under-expression of these channels.
Figures
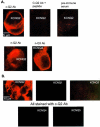
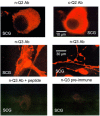
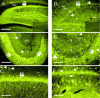

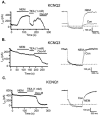

References
-
- BERNHEIM L., BEECH D.J., HILLE B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. - PubMed
-
- BIERVERT C., SCHROEDER B.C., KUBISCH C., BERKOVIC S.F., PROPPING P., JENTSCH T.J., STEINLEIN O.K. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
-
- BROWN D.A., GAHWILER B.H., GRIFFITH W.H., HALLIWELL J.V. Membrane currents in hippocampal neurons. Prog. Brain Res. 1990;83:141–160. - PubMed
-
- CHARLIER C., SINGH N.A., RYAN S.G., LEWIS T.B., REUS B.E., LEACH R.J., LEPPERT M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 1998;18:53–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

