Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle
- PMID: 12466229
- PMCID: PMC1573613
- DOI: 10.1038/sj.bjp.0704988
Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle
Abstract
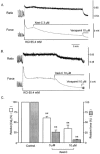
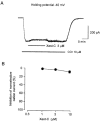
1. Xestospongin-C isolated from a marine sponge, Xestospongia sp., has recently been shown to be a membrane-permeable IP(3) receptor inhibitor. In this study we examined the effects of this compound on smooth muscle from guinea-pig ileum. 2. In guinea-pig ileum permeabilized with alpha-toxin, xestospongin-C (3 microM) inhibited contractions induced by Ca(2+) mobilized from sarcoplasmic reticulum (SR) with IP(3) or carbachol with GTP, but not with caffeine. 3. In intact smooth muscle tissue, xestospongin-C (3-10 microM) inhibited carbachol- and high-K+-induced increases in [Ca(2+)](i) and contractions at sustained phase. 4. It also inhibited voltage-dependent inward Ba(2+) currents in a concentration-dependent manner with an IC(50) of 0.63 microM. Xestospongin-C (3-10 microM) had no effect on carbachol-induced inward Ca(2+) currents via non-selective cation channels; but it did reduce voltage-dependent K+ currents in a concentration-dependent manner with an IC(50) of 0.13 microM. 5. These results suggest that xestospongin-C inhibits the IP(3) receptor but not the ryanodine receptor in smooth muscle SR membrane. In intact smooth muscle cells, however, xestospongin-C appears to inhibit voltage-dependent Ca(2+) and K+ currents at a concentration range similar to that at which it inhibits the IP(3) receptor. Xestospongin-C is a selective blocker of the IP(3) receptor in permeabilised cells but not in cells with intact plasma membrane.
Figures




Similar articles
-
Xestospongin C, a selective and membrane-permeable inhibitor of IP(3) receptor, attenuates the positive inotropic effect of alpha-adrenergic stimulation in guinea-pig papillary muscle.Br J Pharmacol. 2000 Jun;130(3):650-4. doi: 10.1038/sj.bjp.0703358. Br J Pharmacol. 2000. PMID: 10821794 Free PMC article.
-
Purinergic activation of spontaneous transient outward currents in guinea pig taenia colonic myocytes.Am J Physiol Cell Physiol. 2000 Feb;278(2):C352-62. doi: 10.1152/ajpcell.2000.278.2.C352. Am J Physiol Cell Physiol. 2000. PMID: 10666031
-
Regulation of muscarinic cationic current in myocytes from guinea-pig ileum by intracellular Ca2+ release: a central role of inositol 1,4,5-trisphosphate receptors.Cell Calcium. 2004 Nov;36(5):367-86. doi: 10.1016/j.ceca.2004.02.021. Cell Calcium. 2004. PMID: 15451621
-
Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca(2+) pumps.Cell Calcium. 1999 Jul-Aug;26(1-2):9-13. doi: 10.1054/ceca.1999.0047. Cell Calcium. 1999. PMID: 10892566
-
Xestospongin C, a novel blocker of IP3 receptor, attenuates the increase in cytosolic calcium level and degranulation that is induced by antigen in RBL-2H3 mast cells.Br J Pharmacol. 2002 Apr;135(8):1959-66. doi: 10.1038/sj.bjp.0704662. Br J Pharmacol. 2002. PMID: 11959799 Free PMC article.
Cited by
-
Maintenance of homeostatic plasticity at the Drosophila neuromuscular synapse requires continuous IP3-directed signaling.Elife. 2019 Jun 10;8:e39643. doi: 10.7554/eLife.39643. Elife. 2019. PMID: 31180325 Free PMC article.
-
Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles.Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1616-30. doi: 10.1152/ajpheart.00728.2010. Epub 2011 Feb 25. Am J Physiol Heart Circ Physiol. 2011. PMID: 21357503 Free PMC article.
-
Inositol trisphosphate receptors in smooth muscle cells.Am J Physiol Heart Circ Physiol. 2012 Jun 1;302(11):H2190-210. doi: 10.1152/ajpheart.01146.2011. Epub 2012 Mar 23. Am J Physiol Heart Circ Physiol. 2012. PMID: 22447942 Free PMC article. Review.
-
Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry.Cell Calcium. 2008 Feb;43(2):122-41. doi: 10.1016/j.ceca.2007.04.012. Epub 2007 Jun 13. Cell Calcium. 2008. PMID: 17570487 Free PMC article.
-
2-Aminoethoxydiphenyl-borate (2-APB) increases excitability in pyramidal neurons.Cell Calcium. 2009 Mar;45(3):310-7. doi: 10.1016/j.ceca.2008.11.003. Epub 2008 Dec 18. Cell Calcium. 2009. PMID: 19100621 Free PMC article.
References
-
- DE SMET P., PARYS J.B., CALLEWAERT G., WEIDEMA A.F., HILL E., DE SMEDT H., ERNEUX C., SORRENTINO V., MISSIAEN L. Xestospongin-C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium. 1999;26:9–13. - PubMed
-
- FABIATO A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 1981;78:457–497. - PMC - PubMed
-
- GAFNI J., MUNSCH J.A., LAM T.H., CATLIN M.C., COSTA L.G., MOLINSKI T.F., PESSAH L.N. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-triphosphate receptor. Neuron. 1997;19:723–733. - PubMed
-
- HU Q., DESHPANDE S., IRANI K., ZIEGELSTEIN R.C. [Ca2+]i oscillation frequency regulates agonist-stimulated NF-κB transcriptional activity. J. Biol. Chem. 1999;274:33995–33998. - PubMed
-
- HOLDEN C.P., HAUGHEY N.J., DOLHUN B., SHEPEL P.N., NATH A., GEIGER J.D. Diadenosine pentaphosphate increases levels of intracellular calcium in astrocytes by a mechanism involving release from caffeine/ryanodine- and IP3-sensitive stores. J. Neuroscience Res. 2000;59:276–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

